Журнал «Здоровье ребенка» 3 (54) 2014
Вернуться к номеру
Role TLR4, NLRC1/NOD1 AND NF-kB in inflammation gastric mucosa helicobacter pylori infection in children
Авторы: Abaturov A.E., Gerasymenko O.N. - State Establishment «Dnipropetrovsk Medical Academy of the Ministry of Health of Ukraine», Dnipropetrovsk
Рубрики: Педиатрия/Неонатология
Разделы: Клинические исследования
Версия для печати
Introduction. Nonspecific mechanisms of the innate immune system is a defining element of the host defense H.pylori. H.pylori infection-induced modulation of gene expression associated with activation of pattern-recognition receptors (PRR), especially Toll-like receptor (TLR) and Nod-like-receptor (NLR, NLR/NOD) receptors and signaling pathways that lead to activation of transcription factors, including nuclear transcription factor κB (nuclear factor kappa-light-chain-enhancer of activated B cells, NF-κB). Namely adequate action of PRR makes effective eradication of H.pylori, repair damaged tissue, recovery of the patient, while the deficit excitation PRR can cause the development of chronic inflammation and excessive - the emergence of the autoimmune process. One of the main components of the innate immune response to infection H.pylori, the molecular basis underlying the inflammation and morphological changes in mucosal epithelial cells are TLR4, NLR/NOD proteins subfamilies CARD NLRC1/NOD1, transcription factor NF-κB. In connection with this understanding of the molecular mechanisms for the protection of non-specific innate immunity, in particular, the role of TLR4, NLRC1/NOD1, the transcription factor NF-κB - the most important condition for the further improvement of methods of treatment and prevention of chronic H.pylori-associated gastroduodenal diseases in children.
The aim was to study the role of TLR4, NLRC1/NOD1 and NF-κB in inflammation gastric mucosa (GM) in children with chronic inflammatory H. pylori-associated gastroduodenal diseases of the intestinal tract.
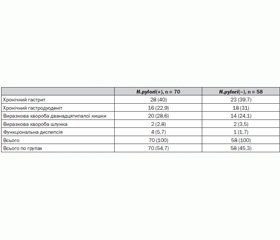
Materials and methods. We observed 128 children with chronic gastroduodenal diseases in the acute stage: 70 (54,7%) children (mean age 14,04 ± 0,34) infected with H.pylori, and 58 (45,3%) - the which H. pylori was not detected (mean age 14,12 ± 0,43). We used molecular genetic methods to determine the level of expression of TLR4, NLRC1/NOD1 biopsy GM and NF-k B in peripheral blood lymphocytes. TLR4, NLRC1/NOD1 gene expression level in the biopsy in real time was performed by polymerase chain reaction, isolation of total RNA from cells of GM was performed using Reagent Kit «RIBO-zol-B» (AmpliSens, Russia). The level of expression of NF-κB+ CD40+ cells was determined using monoclonal antibodies by flow cytometer (EPIX LX-MCL («Beckman Coulter», USA). Determination of the expression of NF-kB in lymphocytes and TLR4 gene expression and NLRC1/NOD1 in the gastric mucosa was carried out in the Research Institute for Genetics and Immunological Grounds of Pathology and Pharmacogenetics, Poltava. Identification of H.pylori-status was performed by rapid urease «Helpil» test and breath «Helik» test («AMA», Russia), the definition of venous blood serum total immunoglobulin (IgM, IgA, IgG) to Ag CagA protein H.pylori by ELISA («Vector-Best», Russia).
Results. We have shown that children infected with H. pylori, show increased levels of gene expression as TLR4, and gene NLRC1/NOD1 (P = 0,01) in the biopsy GM activity while reducing the expression of nuclear factor NF-κB in lymphocytes, in contrast from patients whose disease is not associated with H. pylori infection (P = 0,01). We found that in children a high level of gene expression as TLR4 (activity level 1,9-2,8) and gene NLRC1/NOD1 (activity level ≥ 4,2) in GM biopsy is associated with the risk of complications of chronic gastroduodenal pathology.
It has been shown that children infected with CagA(+) strains of H.pylori was detected significantly lower activity only expression of the transcription factor NF-κB in lymphocytes. Changes in the expression of TLR4, NLRC1/NOD1 in children with infection as CagA(+), and CagA(-) H.pylori strains did not differ significantly. Almost an equivalent expression level of GM in NLRC1/NOD1 children infected CagA(+) and CagA(-) strains of H.pylori, probably indicates that peptidoglycan (PGN) of H. pylori penetrate epithelial GM not only by transport system type IV secretion, but also due to the outer membrane vesicles transport the pathogen.
Conclusion. 1. In patients infected with H.pylori, there is an increase in expression level of the gene as TLR4, and NLRC1/NOD1 gene in biopsies of the GM with reduced activity of the expression of nuclear factor NF-κB in lymphocytes, as opposed to children whose disease was not associated with H.pylori-infection.
2. Changes in the activity of nonspecific protection components such as the expression of TLR4, NLRC1/NOD1 and NF-κB in children with chronic gastroduodenal pathology when infected by cytotoxic CagA (+) strains of H.pylori differed from their responses when infected non cytotoxic CagA(-) strains of H.pylori only significantly lower activity of the NF-κB in lymphocytes.
3. We propose a model of participation of non-specific mechanisms of innate immunity in the development of chronic inflammation of the gastric mucosa in children infected with CagA(+) and CagA(-) strains of H.pylori.