Газета «Новости медицины и фармации» Неврология. Нейрохирургия. Психиатрия (549) 2015 (тематический номер)
Вернуться к номеру
Guidelines for the Management of Spontaneous Intracerebral Hemorrhage. A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association
Авторы: J. Claude Hemphill III, MD, MAS, FAHA, Chair; Steven M. Greenberg, MD, PhD, Vice-Chair;
Craig S. Anderson, MD, PhD; Kyra Becker, MD, FAHA; Bernard R. Bendok, MD, MS, FAHA;
Mary Cushman, MD, MSc, FAHA; Gordon L. Fung, MD, MPH, PhD, FAHA; Joshua N. Goldstein, MD, PhD, FAHA; R. Loch Macdonald, MD, PhD, FRCS; Pamela H. Mitchell, RN, PhD, FAHA; Phillip A. Scott, MD, FAHA; Magdy H. Selim, MD, PhD; Daniel Woo, MD, MS; от лица Совета по инсульту, Совета по уходу за пациентами с сердечно-сосудистыми заболеваниями и инсультом, а также Совета по клинической кардиологии Американской кардиологической ассоциации
Рубрики: Неврология, Психиатрия
Разделы: Справочник специалиста
Версия для печати
Статья опубликована на с. 47-63
The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.
Endorsed by the American Association of Neurological Surgeons, the Congress of Neurological Surgeons, and the Neurocritical Care Society.
Endorsed by the American Association of Neurological Surgeons, the Congress of Neurological Surgeons, and the Neurocritical Care Society.
Spontaneous, nontraumatic intracerebral hemorrhage (ICH) remains a significant cause of morbidity and mortality throughout the world. Although ICH has traditionally lagged behind ischemic stroke and aneurysmal subarachnoid hemorrhage in terms of evidence from clinical trials to guide management, the past decade has seen a dramatic increase in studies of ICH intervention. Population-based studies show that most patients present with small ICHs that are readily survivable with good medical care [1]. This suggests that excellent medical care likely has a potent, direct impact on ICH morbidity and mortality. This guideline serves several purposes. One is to provide an update to the last American Heart Association/American Stroke Association ICH guideline, published in 2010, incorporating the results of new studies published in the interim [2]. Another equally important purpose is to remind clinicians of the importance of their care in determining ICH outcome and to provide an evidence-based framework for that care.
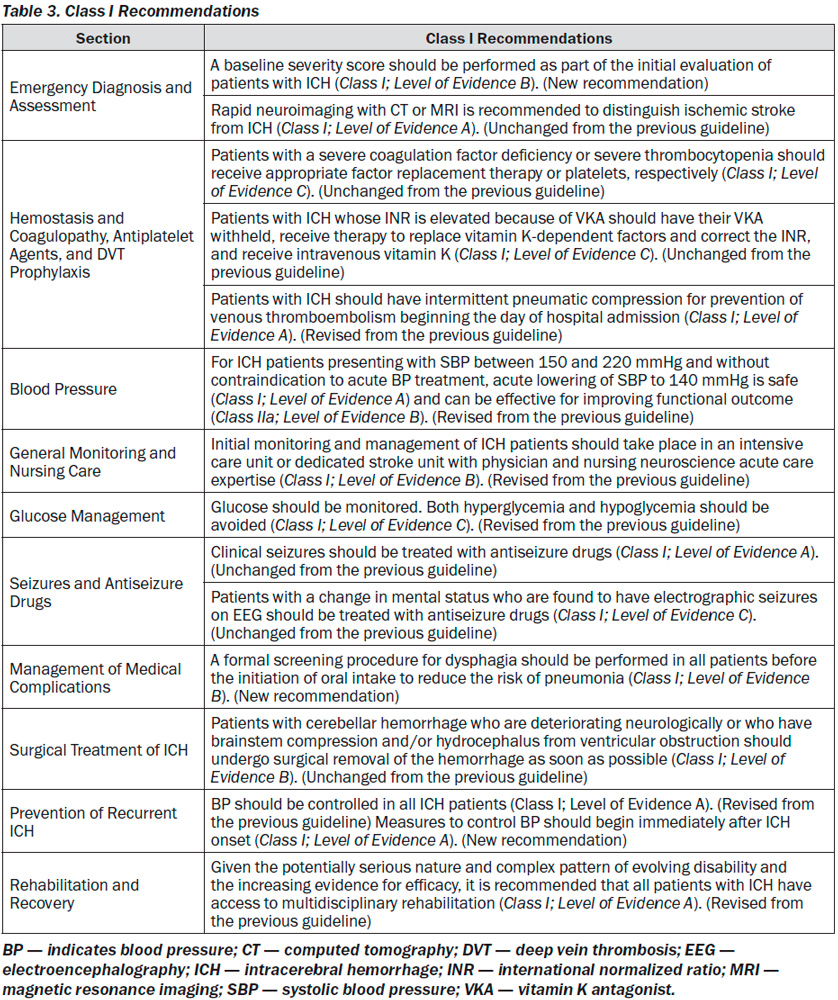
All Class I recommendations are listed in Table 3.
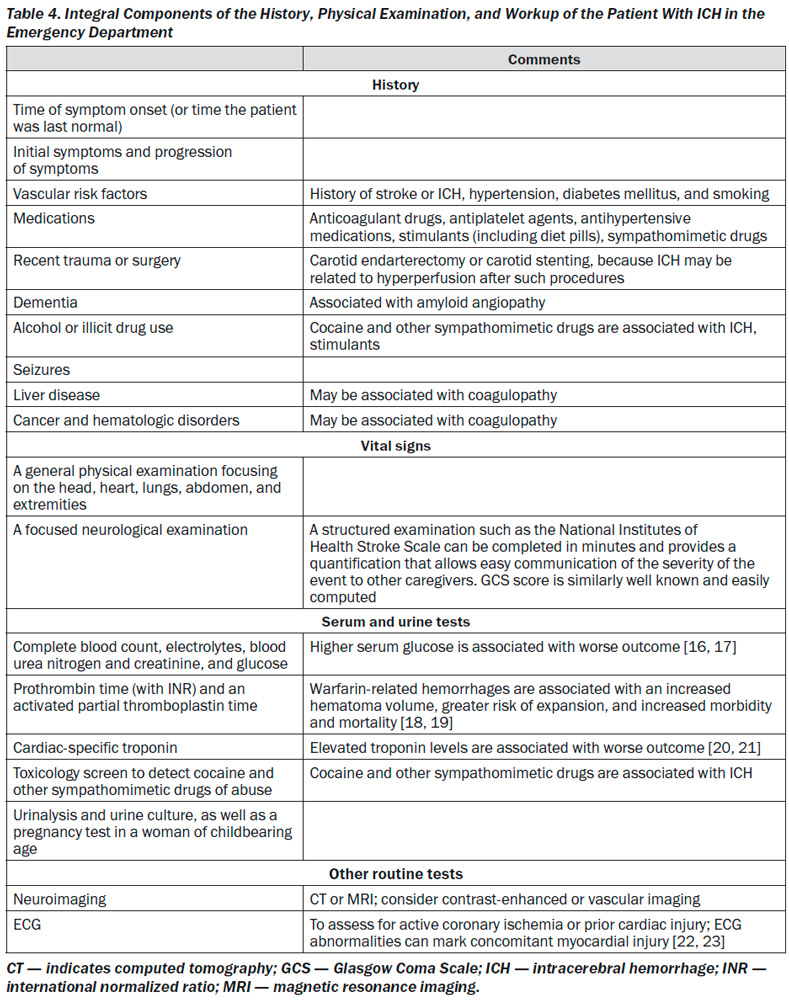
ICH is a medical emergency. Rapid diagnosis and attentive management of patients with ICH is crucial, because early deterioration is common in the first few hours after ICH onset. More than 20 % of patients will experience a decrease in the Glasgow Coma Scale (GCS) of 2 or more points between the prehospital emergency medical services (EMS) assessment and the initial evaluation in the emergency department (ED) [6]. Furthermore, another 15 to 23 % of patients demonstrate continued deterioration within the first hours after hospital arrival [7, 8]. The risk for early neurological deterioration and the high rate of poor long-term outcomes underscore the need for aggressive early management.
Neuroimaging
The abrupt onset of focal neurological symptoms is presumed to be vascular in origin until proven otherwise; however, it is impossible to know whether symptoms are caused by ischemia or hemorrhage on the basis of clinical characteristics alone. Vomiting, systolic BP (SBP) > 220 mmHg,
severe headache, coma or decreased level of consciousness, and symptom progression over minutes or hours all suggest ICH, although none of these findings are specific; neuroimaging is thus mandatory [40]. CT and magnetic resonance imaging (MRI) are both reasonable for initial evaluation. CT is very sensitive for identifying acute hemorrhage and is considered the gold standard; gradient echo and T2* susceptibility-weighted MRI are as sensitive as CT for detection of acute hemorrhage and are more sensitive for identification of prior hemorrhage [41, 42]. Time, cost, proximity to the ED, patient tolerance, clinical status, and MRI availability may, however, preclude emergent MRI in many cases [43].
The high rate of early neurological deterioration after ICH is related in part to active bleeding that may proceed for hours after symptom onset. Hematoma expansion tends to occur early after ICH and increases risk of poor functional outcome and death [7, 44–49]. Among patients undergoing head CT within 3 hours of ICH onset, 28 to 38 % have hematoma expansion of greater than one third of the initial hematoma volume on follow-up CT [7, 45]. As such, the identification of patients at risk for hematoma expansion is an active area of research. CT angiography (CTA) and contrast-enhanced CT may identify patients at high risk of ICH expansion based on the presence of contrast within the hematoma, often termed a spot sign [50–54]. A larger number of contrast spots suggests even higher risk of expansion [55, 56].
Early diagnosis of underlying vascular abnormalities can both influence clinical management and guide prognosis in ICH patients. Risk factors for underlying vascular abnormalities are age < 65 years, female sex, nonsmoker, lobar ICH, intraventricular extension, and absence of a history of hypertension or coagulopathy [57, 58]. MRI, magnetic resonance angiography, magnetic resonance venography, and CTA or CT venography can identify specific causes of hemorrhage, including arteriovenous malformations, tumors, moyamoya, and cerebral vein thrombosis [59–61]. CTA has been more widely studied and is highly sensitive and specific for detecting vascular abnormalities [58, 62–64]. A catheter angiogram may be considered if clinical suspicion is high or noninvasive studies are suggestive of an underlying lesion [65]. Radiological evidence suggestive of vascular abnormalities as causative for ICH can include the presence of subarachnoid hemorrhage, enlarged vessels or calcifications along the margins of the ICH, hyperattenuation within a dural venous sinus or cortical vein along the presumed venous drainage path [56], unusual hematoma shape, pre-sence of edema out of proportion to the time of presumed ICH, an unusual hemorrhage location, and the presence of other abnormal structures in the brain (like a mass). Patients with lobar hemorrhage location, age < 55 years, and no history of hypertension have a higher likelihood of identification of a secondary cause of ICH from additional MRI beyond noncontrast CT [66]. An magnetic resonance venography or CT venography should be performed if hemorrhage location, relative edema volume, or abnormal signal in the cerebral sinuses on routine neuroimaging suggests cerebral vein thrombosis.
In summary, ICH is a medical emergency that should be diagnosed and managed promptly. Hematoma expansion and early deterioration are common within the first few hours after onset.
Emergency Diagnosis and Assessment: Recommendations
1. A baseline severity score should be performed as part of the initial evaluation of patients with ICH (Class I; Level of Evidence B). (New recommendation)
2. Rapid neuroimaging with CT or MRI is re-commended to distinguish ischemic stroke from ICH (Class I; Level of Evidence A). (Unchanged from the previous guideline)
3. CTA and contrast-enhanced CT may be considered to help identify patients at risk for hematoma expansion (Class IIb; Level of Evidence B), and CTA, CT venography, contrast-enhanced CT, contrastenhanced MRI, magnetic resonance angiography and magnetic resonance venography, and catheter angiography can be useful to evaluate for underlying structural lesions inclu-ding vascular malformations and tumors when there is clinical or radiological suspicion (Class IIa; Level of Evidence B). (Unchanged from the previous guideline)
Medical Treatment for ICH
Hemostasis and Coagulopathy, Antiplatelets, and Deep Vein Thrombosis Prophylaxis
Underlying hemostatic abnormalities can contribute to ICH. Patients at risk include those taking oral anticoagulant drugs (OACs), antiplatelet agents, those with acquired or congenital coagulation factor deficiencies, and those with inherited or acquired qualitative or quantitative platelet abnormalities. Patients taking OACs constitute 12 to 20 % of patients with ICH [67–69], a rate that has increased with the aging population and increased use of anticoagulant drugs in recent decades [67, 70]. Vitamin K antagonists (VKAs) such as warfarin are the most frequently prescribed OAC, but new agents that do not require laboratory monitoring and do not necessarily prolong coagulation screening tests are being increasingly used, including dabigatran [71], rivaroxaban [72], and apixaban [73]. These new agents appear to be associated with a lower risk of ICH than VKAs [74]. It is important that providers caring for ICH patients recognize the use of antithrombotic drugs or of an underlying coagulopathy in the initial evaluation of patients with ICH, so that the treatment strategy can include appropriate interventions. For patients with a known coagulation factor deficiency or platelet disorder, replacement of the appropriate factor or platelets, often with the assistance of a consultant hematologist, is indicated. If spontaneous ICH occurs in a patient undergoing an intravenous heparin infusion, then protamine sulfate can be given by intravenous injection at a dose of 1 mg per 100 U of heparin (maximum dose 50 mg), with adjustment based on time elapsed since discontinuation of heparin infusion [75]. Similar dosing can be used in patients who are receiving low-molecular-weight heparin; however, reversal may be incomplete [39].
VKA-Related ICH
Guidelines exist for reversal of OACs [76]. For ICH patients taking VKA, rapid correction of the international normalized ratio (INR) is recommended [76, 77]. Fresh frozen plasma (FFP), along with vitamin K, has been the mainstay of treatment in the United States for years, but more recently, prothrombin complex concentrates (PCCs), the activated PCC FEIBA (factor VIII inhibitor bypassing activity), and recombinant activated factor VIIa (rFVIIa) have emerged as potential therapies. Administration of intravenous vitamin K alone is insufficient for reversal in the first hours but should be part of all acute VKA reversal strategies in a dose of 5 to 10 mg, usually given slowly via the intravenous route. Onset of action begins by 2 hours and is maximal at ≈ 24 hours if liver function is normal [78]. FFP administration requires thawing and cross matching, carries a risk of allergic and infectious transfusion reactions, and often requires large volumes for full INR correction. Likelihood of INR correction at 24 hours was linked to time to FFP administration in 1 study, although 17 % of patients still did not have an INR < 1.4 by this time, which suggests that FFP administered in this manner may be insufficient for rapid correction of coagulopathy [79]. Shortcomings of FFP have led to interest in alternative agents for VKA reversal. PCCs are plasma-derived factor concentrates originally developed to treat factor IX deficiency (hemophilia B). Threefactor PCC contains factors II, IX, and X whereas 4-factor PCC also contains factor VII. PCC does not require cross matching, can be reconstituted and administered rapidly in a small volume (20–40 mL), and has been processed to inactivate infectious agents. Several studies have shown that PCCs rapidly normalize the INR (within minutes) in patients taking VKAs [80–82]. Although nonrando-mized retrospective reviews and a small case-control study have shown more rapid correction of INR with vitamin K and PCC than vitamin K and FFP, none have clearly demonstrated an improvement in patient clinical outcome with PCC [83–85]. In 1 randomized trial comparing the use of a PCC (Konyne) to supplement FFP versus FFP alone in patients with VKA-related ICH, those who were given FFP alone received a higher volume of FFP and developed more adverse events, primarily attributable to fluid overload [86]. PCCs may increase the risk of thrombotic complications, although this risk appears low [80]. In 2013, the first large phase 3 rando-mized controlled trial demonstrated noninferiority of 4-factor PCC to FFP for urgent reversal of warfarin in a cohort of 202 patients with acute bleeding (24 of whom had intracranial hemorrhage) [87]. In this study, the rate of achieving an INR < 1.3 within 30 minutes of completing therapy was 62.2 % for PCC and 9.6 % for FFP. Thromboembolic event rates were similar (7.8 % with PCC and 6.4 % with FFP), and fluid overload was more common with FFP (12.8 % versus 4.9 %). Analogous randomized trials have not been performed to directly evaluate 3-factor and 4-factor PCCs against each other. Additionally, the specific INR target for VKA correction in OAC-related ICH is unclear, with various stu-dies cited here and elsewhere using targets ran-ging from < 1.5 [88].
rFVIIa, licensed to treat hemophilia patients with high titer inhibitors or congenital factor VII deficiency, has garnered attention as a potential treatment for spontaneous and OAC-associa-ted ICH. Although rFVIIa can rapidly norma-lize INR in the setting of VKA-associated ICH [89–93], it does not replenish all of the vitamin K-dependent factors and may not restore thrombin generation as effectively as PCCs [94]. Thus, rFVIIa is not currently recommended for routine use in warfarin reversal [95].
New Anticoagulant Medication — Related ICH
There are no randomized trials of reversing agents for newer anticoagulants among patients with ICH or other major bleeding complications, and because these agents have only been avai-lable for a few years, experience with reversal is limited. Currently available agents in the United States (dabigatran, rivaroxaban, and apixaban) have relatively short half-lives ranging from 5 to 15 hours. Evaluation of the activated partial thromboplastin time and prothrombin time and consultation with a hematologist are reasonable to individualize care. Potential reversal strategies using FEIBA, other PCCs, or rFVIIa might be considered. FFP is of unclear utility, and vitamin K is not useful. It has been suggested that FEIBA or rFVIIa may be better for the direct thrombin inhibitor dabigatran, whereas other PCCs may be better for the factor Xa inhibitors rivaroxaban and apixaban [96–99], but these data are preliminary. Activated charcoal can be used if the most recent dose of dabigatran, apixaban, or rivaroxaban was taken within the previous couple of hours [100]. Hemodialysis has been noted as an option for dabigatran, but less so for rivaroxaban or apixaban because these are more highly protein bound [90]. Specific antidotes for these medications are in early clinical development [101].
Antiplatelet Medication — Related ICH
Studies addressing the effect of prior antiplatelet agent use or platelet dysfunction on ICH growth and outcome have found conflicting results. Reported antiplatelet agent use was not associated with hematoma expansion or clinical outcome in the placebo group of an ICH neuroprotective study [102]. Others have suggested that platelet dysfunction as measured by platelet function assays may be associated with hematoma expansion and clinical outcome [103, 104]. Platelet function monitoring could be helpful in assessing exposure to antiplatelet medications and guiding hemostatic interventions, but this approach has not been fully studied. A case series of 45 ICH patients receiving platelet transfusion at the discretion of their physician demonstrated improved platelet reactivity after transfusion with the VerifyNow-ASA assay [105]. Subgroup analysis in those at high risk of hemorrhage growth suggested that platelet transfusion within 12 hours of symptom onset was associated with smaller final hemorrhage outcome and independence at 3 months. Two randomized controlled trials are ongoing to evaluate the effectiveness of platelet transfusion in ICH patients taking antiplatelet agents [106, 107].
rFVIIa in ICH Not Related to Anticoagulant Agents
rFVIIa has also been tested in patients with non-OAC ICH. Although a phase 2 randomized trial showed that treatment with rFVIIa within 4 hours after ICH onset limited hematoma growth and improved clinical outcome relative to placebo, a subsequent phase 3 trial did not find clinical benefit [108, 109]. Use of rFVIIa was associated with an increased frequency of thromboembolic events compared with placebo (7 % versus 2 %) in the phase 2 trial and significantly more arterial events in the phase 3 trial. It remains to be determined whether rFVIIa might benefit a particular subset of patients with ICH, but currently its benefits in ICH patients, whether or not they are taking an OAC, remain unproven.
Thromboprophylaxis in ICH Patients
Patients with ICH have a high risk of thromboembolic disease [110]. Women and blacks may be at greater risk [110–112]. In a randomized trial of 151 ICH patients, intermittent pneumatic compression together with elastic stockings reduced the occurrence of asymptomatic deep vein thrombosis (DVT) after ICH compared with elastic stockings alone (4.7 % versus 15.9 %) [113]. The CLOTS trials (Clots in Legs or Stockings After Stroke) consisted of 3 different randomized trials (CLOTS 1, 2, and 3) that assessed several different treatments, including graduated compression stockings versus none, thigh-high graduated compression stockings versus calf-high stockings, and intermittent pneumatic compression versus none [114–117]. CLOTS 1 enrolled 2518 stroke patients (232 with ICH) and found that thigh-high compression stockings did not reduce DVT, pulmonary embolism (PE), or death [115]. CLOTS 2 found that DVT was more common in patients who had below-knee graduated compression stockings than in those with thighhigh graduated compression stockings [114]. Finally, CLOTS 3 enrolled 2876 patients (376 with ICH) and found that intermittent pneumatic compression begun as early as the day of hospital admission reduced the occurrence of proximal DVT, with the effect being particularly prominent in patients with hemorrhagic stroke (6.7 % versus 17.0 %, odds ratio [OR] 0.36; 95% confidence interval [CI] 0.17–0.75) [116]. A meta-analysis of anticoagulant drugs for thromboprophylaxis that included 1000 ICH patients from 4 trials (2 randomized) and evaluated the early use of enoxaparin or heparin (from 1 to 6 days after admission) found a reduction in PE (1.7 % versus 2.9 %; relative risk [RR] 0.37; 95% CI 0.17–0.80), a nonsigni-ficant reduction in mortality (16.1 % versus 20.9 %; RR 0.76; 95% CI 0.57–1.03), but no difference in DVT (4.2 % versus 3.3 %; RR 0.77; 95% CI 0.44–1.34) or hematoma enlargement (8.0 % versus 4.0 %; RR 1.42; 95% CI 0.57–3.53) [118].
ICH patients who develop DVT or PE may be considered for full systemic anticoagulation or placement of an inferior vena cava (IVC) filter. Given the generally accepted recurrence rate of nonfatal PE is 12 to 15 % in nontreated patients (not specific to ICH), observation alone is not recommended. Only very limited information is available to guide decision making on IVC filter placement versus anticoagulation, as well as the optimal anticoagulation regimen [119]. Conside-rations include the posthemorrhage date on which DVT/PE is diagnosed, documentation of stable hematoma size on neuroimaging, lobar versus deep hematoma location, and the practical ability to remove an IVC filter at a later date. General guidelines for the use of IVC filters in the setting of acute DVT suggest a conventional course of anticoagulant therapy if the risk of bleeding resolves; however, these are not ICH specific [120].
Hemostasis and Coagulopathy, Antiplatelet Agents, and DVT Prophylaxis: Recommendations
1. Patients with a severe coagulation factor deficiency or severe thrombocytopenia should receive appropriate factor replacement therapy or platelets, respectively (Class I; Level of Evidence C). (Unchanged from the previous guideline)
2. Patients with ICH whose INR is elevated because of VKA should have their VKA withheld, receive therapy to replace vitamin K-dependent factors and correct the INR, and receive intravenous vitamin K (Class I; Level of Evidence C). PCCs may have fewer complications and correct the INR more rapidly than FFP and might be considered over FFP (Class IIb; Level of Evidence B). rFVIIa does not replace all clotting factors, and although the INR may be lowered, clotting may not be restored in vivo; therefore, rFVIIa is not recommended for VKA reversal in ICH (Class III; Level of Evidence C). (Revised from the previous guideline)
3. For patients with ICH who are taking dabigatran, rivaroxaban, or apixaban, treatment with FEIBA, other PCCs, or rFVIIa might be consi-dered on an individual basis. Activated charcoal might be used if the most recent dose of dabigatran, apixaban, or rivaroxaban was taken < 2 hours earlier. Hemodialysis might be considered for dabigatran (Class IIb; Level of Evidence C). (New recommendation)
4. Protamine sulfate may be considered to reverse heparin in patients with acute ICH (Class IIb; Level of Evidence C). (New recommendation)
5. The usefulness of platelet transfusions in ICH patients with a history of antiplatelet use is uncertain (Class IIb; Level of Evidence C). (Revised from the previous guideline)
6. Although rFVIIa can limit the extent of hematoma expansion in noncoagulopathic ICH patients, there is an increase in thromboembolic risk with rFVIIa and no clear clinical benefit in unselected patients. Thus, rFVIIa is not recommended (Class III; Level of Evidence A). (Unchanged from the previous guideline)
7. Patients with ICH should have intermittent pneumatic compression for prevention of venous thromboembolism beginning the day of hospital admission (Class I; Level of Evidence A). Graduated compression stockings are not beneficial to reduce DVT or improve outcome (Class III; Level of Evidence A). (Revised from the previous guideline)
8. After documentation of cessation of bleeding, lowdose subcutaneous low-molecular-weight heparin or unfractionated heparin may be considered for prevention of venous thromboembolism in patients with lack of mobility after 1 to 4 days from onset (Class IIb; Level of Evidence B). (Unchanged from the previous guideline)
9. Systemic anticoagulation or IVC filter placement is probably indicated in ICH patients with symptomatic DVT or PE (Class IIa; Level of Evidence C). The decision between these 2 options should take into account several factors, including time from hemorrhage onset, hematoma stability, cause of hemorrhage, and overall patient condition (Class IIa; Level of Evidence C). (New recommendation)
BP and Outcome in ICH
Elevated BP is very common in acute ICH [121, 122] because of a variety of factors, including stress, pain, increased ICP, and premorbid acute or persistent elevations in BP. High SBP is associated with greater hematoma expansion, neurological deterioration, and death and dependency after ICH [122–124]. Compared with ischemic stroke, in which consistent U- or J-shaped associations between SBP nadir of 140 and 150 mmHg and poor outcome have been shown [125], only 1 study of ICH has shown a poor outcome at low SBP levels (< 140 mmHg) [126].
BP: Recommendations
1. For ICH patients presenting with SBP between 150 and 220 mmHg and without contraindication to acute BP treatment, acute lowering of SBP to 140 mmHg is safe (Class I; Level of Evidence A) and can be effective for improving functional outcome (Class IIa; Level of Evidence B). (Revised from the previous guideline)
2. For ICH patients presenting with SBP > 220 mmHg, it may be reasonable to consider aggressive reduction of BP with a continuous intravenous infusion and frequent BP monitoring (Class IIb; Level of Evidence C). (New recommendation)
General Monitoring and Nursing Care: Recommendation
1. Initial monitoring and management of ICH patients should take place in an intensive care unit or dedicated stroke unit with physician and nursing neuroscience acute care expertise (Class I; Level of Evidence B). (Revised from the previous guideline)
Glucose Management: Recommendation
1. Glucose should be monitored. Both hyperglycemia and hypoglycemia should be avoided (Class I; Level of Evidence C). (Revised from the previous guideline)
Temperature Management: Recommendation
1. Treatment of fever after ICH may be reasonable (Class IIb; Level of Evidence C). (New recommendation)
Seizures and Antiseizure Drugs: Recommendations
1. Clinical seizures should be treated with antiseizure drugs (Class I; Level of Evidence A). (Unchanged from the previous guideline)
2. Patients with a change in mental status who are found to have electrographic seizures on EEG should be treated with antiseizure drugs (Class I; Level of Evidence C). (Unchanged from the previous guideline)
3. Continuous EEG monitoring is probably indicated in ICH patients with depressed mental status that is out of proportion to the degree of brain injury (Class IIa; Level of Evidence C). (Revised from the previous guideline)
4. Prophylactic antiseizure medication is not recommended (Class III; Level of Evidence B). (Unchanged from the previous guideline)
Management of Medical Complications: Recommendations
1. A formal screening procedure for dysphagia should be performed in all patients before the initiation of oral intake to reduce the risk of pneumonia (Class I; Level of Evidence B). (New recommendation)
2. Systematic screening for myocardial ischemia or infarction with electrocardiogram and cardiac enzyme testing after ICH is reasonable (Class IIa; Level of Evidence C). (New recommendation)
ICP Monitoring and Treatment: Recommendations
1. Ventricular drainage as treatment for hydrocephalus is reasonable, especially in patients with decreased level of consciousness (Class IIa; Level of Evidence B). (Revised from the previous guideline)
2. Patients with a GCS score of ≤ 8, those with clinical evidence of transtentorial herniation, or those with significant IVH or hydrocephalus might be considered for ICP monitoring and treatment. A CPP of 50 to 70 mmHg may be reasonable to maintain depending on the status of cerebral autoregulation (Class IIb; Level of Evidence C). (Unchanged from the previous guideline)
3. Corticosteroids should not be administered for treatment of elevated ICP in ICH (Class III; Level of Evidence B). (New recommendation)
IVH: Recommendations
1. Although intraventricular administration of rtPA in IVH appears to have a fairly low complication rate, the efficacy and safety of this treatment are uncertain (Class IIb; Level of Evidence B). (Revised from the previous recommendation)
2. The efficacy of endoscopic treatment of IVH is uncertain (Class IIb; Level of Evidence B). (New recommendation)
Surgical Treatment of ICH: Recommendations
1. Patients with cerebellar hemorrhage who are deteriorating neurologically or who have brainstem compression and/or hydrocephalus from ventricular obstruction should undergo surgical removal of the hemorrhage as soon as possible (Class I; Level of Evidence B). Initial treatment of these patients with ventricular drainage rather than surgical evacuation is not recommended (Class III; Level of Evidence C). (Unchanged from the previous guideline)
2. For most patients with supratentorial ICH, the usefulness of surgery is not well established (Class IIb; Level of Evidence A). (Revised from the previous guideline) Specific exceptions and potential subgroup considerations are outlined below in recommendations 3 through 6.
3. A policy of early hematoma evacuation is not clearly beneficial compared with hematoma evacuation when patients deteriorate (Class IIb; Level of Evidence A). (New recommendation)
4. Supratentorial hematoma evacuation in deteriorating patients might be considered as a life-saving measure (Class IIb; Level of Evidence C). (New recommendation)
5. DC with or without hematoma evacuation might reduce mortality for patients with supratentorial ICH who are in a coma, have large hematomas with significant midline shift, or have elevated ICP refractory to medical management (Class IIb; Level of Evidence C). (New recommendation)
6. The effectiveness of minimally invasive clot evacuation with stereotactic or endoscopic aspiration with or without thrombolytic usage is uncertain (Class IIb; Level of Evidence B). (Revised from the previous guideline)
Outcome Prediction and Withdrawal of Technological Support: Recommendation
1. Aggressive care early after ICH onset and postponement of new DNAR orders until at least the second full day of hospitalization is probably recommended (Class IIa; Level of Evidence B). Patients with preexisting DNAR orders are not included in this recommendation. Current prognostic models for individual patients early after ICH are biased by failure to account for the influence of withdrawal of support and early DNAR orders. DNAR status should not limit appropriate medical and surgical interventions unless otherwise explicitly indicated (Class III; Level of Evidence C). (Revised from the previous guideline)
Risk Factors
Hypertension, older age, and location of the initial hemorrhage (deep versus lobar) are important risk factors for ICH recurrence [269, 271]. High BP is associated with an increase in the recurrence of both deep and lobar hemor-rhages [271]. Increased risk in the elderly is attributed to a higher prevalence of cerebral amyloid angiopathy (CAA) and increased use of antithrombotic medications with accumulating comorbidities [272]. CAA is a recognized risk factor for recurrent ICH, particularly in lobar locations [271]. Carriers of the apolipoprotein E ε2 or ε4 alleles [273], patients with previous ICH before the presenting ICH [274], and patients with a greater number of microbleeds (particularly microbleeds in lobar brain locations) on gradient echo MRI appear to be at higher risk for ICH recurrence [272, 275]. In whites, most of the initial and recurrent hemorrhages tend to be lobar, whereas deep hemorrhages (both initial and recurrent) are more common in Asians [269, 276]. A history of ischemic stroke, particularly of the small-vessel «lacunar» type, which shares a common pathogenesis with ICH, might also be a predictor of ICH recurrence [277, 278].
Prevention of Recurrent ICH: Recommendations
1. When stratifying a patient’s risk for recurrent ICH may affect management decisions, it is reasonable to consider the following risk factors for ICH recurrence: 1) lobar location of the initial ICH; 2) older age; 3) presence and number of microbleeds on gradient echo MRI; 4) ongoing anticoagulation; and 5) presence of apolipoprotein E ε2 or ε4 alleles (Class IIa; Level of Evidence B). (Revised from the previous guideline)
2. BP should be controlled in all ICH patients (Class I; Level of Evidence A). (Revised from the previous guideline) Measures to control BP should begin immediately after ICH onset (Class I; Level of Evidence A). (New recommendation) A long-term goal of BP < 130 mmHg systolic and 80 mmHg diastolic is reasonable (Class IIa; Level of Evidence B). (New recommendation)
3. Lifestyle modifications, including avoi-dance of alcohol use greater than 2 drinks per day, tobacco use, and illicit drug use, as well as treatment of obstructive sleep apnea, are probably beneficial (Class IIa; Level of Evidence B). (Revised from previous guideline)
4. Avoidance of long-term anticoagulation with warfarin as a treatment for nonvalvular atrial fibrillation is probably recommended after warfarin-associated spontaneous lobar ICH because of the relatively high risk of recurrence (Class IIa; Level of Evidence B). (Unchanged from the previous guideline)
5. Anticoagulation after nonlobar ICH and antiplatelet monotherapy after any ICH might be considered, particularly when there are strong indications for these agents (Class IIb; Level of Evidence B). (Revised from the previous guideline)
6. The optimal timing to resume oral anticoagulation after anticoagulant-related ICH is uncertain. Avoidance of oral anticoagulation for at least 4 weeks, in patients without mechanical heart valves, might decrease the risk of ICH recurrence (Class IIb; Level of Evidence B). (New recommendation) If indicated, aspirin monotherapy can probably be restarted in the days after ICH, although the optimal timing is uncertain (Class IIa; Level of Evidence B). (New recommendation)
7. The usefulness of dabigatran, rivaroxaban, or apixaban in patients with atrial fibrillation and past ICH to decrease the risk of recurrence is uncertain (Class IIb; Level of Evidence C). (New recommendation)
8. There are insufficient data to recommend restrictions on the use of statins in ICH patients (Class IIb; Level of Evidence C). (Unchanged from the previous guideline)
Rehabilitation and Recovery: Recommendations
1. Given the potentially serious nature and complex pattern of evolving disability and the increasing evidence for efficacy, it is recommended that all patients with ICH have access to multidisciplinary rehabilitation (Class I; Level of Evidence A). (Revised from the previous guideline)
2. Where possible, rehabilitation can be beneficial when begun as early as possible and continued in the community as part of a well-coordinated («seamless») program of accelerated hospital discharge and home-based resettlement to promote ongoing recovery (Class IIa; Level of Evidence B). (Unchanged from the previous guideline)
In Abridged Form