Резюме
Актуальність. Стандартна обробка нового випадку туберкульозу з множинною лікарською стійкістю (МЛС ТБ) відповідно до рекомендацій ВООЗ у Республіці Молдова проводиться з 2005 року, що свідчить про низьку ефективність лікування. Фактично частота успішного лікування збільшилась через виключення пацієнтів з МЛС ТБ із загальної когорти. Основний показник у пацієнтів з низьким результатом ефективності лікування становлять невдалі й втрачені для наступної оцінки випадки. Мета дослідження полягала в тому, щоб дати оцінку впливу полі- і мультирезистентності на результати лікування туберкульозу. Матеріали та методи. Проведене ретроспективне вибіркове описове дослідження, орієнтоване на соціальні, демографічні, економічні й епідеміологічні особливості, ведення хворих, діагностичні радіологічні аспекти й мікробіологічні характеристики 187 пацієнтів із туберкульозом легень, зареєстрованих у 2013–2015 рр. Були ідентифіковані дві групи: 1-ша група (61 пацієнт) — виділені полістійкі штами МБТ традиційними методами культивування; 2-га група (126 пацієнтів) — хворі з варіантом МЛС ТБ. Результати. Встановлено, що полірезистентність була виявлена частіше в нових випадках МЛС ТБ у двох третин пацієнтів, які відхилялись від схеми лікування. Не виявлено різниці в розподілі за статтю і віком, соціальними, економічними, освітніми характеристиками. Оцінка керування захворюванням показала схожу частку пацієнтів, виявлених лікарями загальної практики і фахівцями, з низьким рівнем скринованих груп високого ризику. Усі пацієнти з полірезистентної групи почали стандартне лікування лікарсько-чутливого туберкульозу до початку тестування лікарської чутливості, а одна третина з групи хворих на МЛС ТБ лікувалась з початку терапії за ДОТС-Плюс. Найвищий показник успішного лікування був виявлений у нових підгрупах обох груп, а більш висока частота померлих пацієнтів була в підгрупах з повторним лікуванням. Такий низький рівень пацієнтів підвищує резистентність. Висновки. Рання діагностика, тестування чутливості до лікарських засобів і підвищення обізнаності про дотримання режиму лікування покращує результат захворювання.
Актуальность. Стандартная обработка нового случая туберкулеза с множественной лекарственной устойчивостью (МЛУ ТБ) в соответствии с рекомендациями ВОЗ в Республике Молдова проводится с 2005 года, что свидетельствует о низкой эффективности лечения. Фактически частота успешного лечения увеличилась из-за исключения пациентов с МЛУ ТБ из общей когорты. Основной показатель пациентов с низким исходом эффективности лечения представлен неудавшимися и потерянными для последующей оценки случаями. Цель исследования состояла в том, чтобы дать оценку влияния поли- и мультирезистентности на результаты лечения туберкулеза. Материалы и методы. Проведено ретроспективное выборочное описательное исследование, ориентированное на социальные, демографические, экономические и эпидемиологические особенности, ведение больных, диагностические радиологические аспекты и микробиологические характеристики 187 пациентов с туберкулезом легких, зарегистрированных в 2013–2015 гг. Были идентифицированы две группы: 1-я группа (61 пациент) — выделены полиустойчивые штаммы МТБ традиционными методами культивирования, 2-я группа (126 пациентов) — больные с вариантом МЛУ ТБ. Результаты. Установлено, что полирезистентность была выявлена чаще в новых случаях и МЛУ ТБ у двух третей пациентов, которые отклонялись от схемы лечения. Не выявлено различий в распределении по полу и возрасту, социальных, экономических, образовательных характеристиках. Оценка управления заболеванием показала сходную долю пациентов, выявленных врачами общей практики и специалистами, с низким уровнем скринированных групп высокого риска. Все пациенты из полирезистентной группы начали стандартное лечение лекарственно-чувствительного туберкулеза до начала тестирования лекарственной чувствительности, а одна треть из группы больных МЛУ ТБ лечилась с начала терапии ДОТС-Плюс. Наивысший показатель успеха был выявлен в новых подгруппах обеих групп, а более высокая частота умерших пациентов была установлена в подгруппах с повторным лечением. Подобный низкий уровень пациентов повышает резистентность. Выводы. Ранняя диагностика, тестирование чувствительности к лекарственным средствам и повышение осведомленности о соблюдении режима лечения улучшают исход заболевания.
Background. The standard treatment of a new case of multidrug-resistant tuberculosis (MDR-TB) according to WHO recommendations in the Republic of Moldova is performed since 2005 showing a low treatment succes. Actually the treatment success rate increased due to excluding of MDR-TB patients from the general cohort. The major rate of patients with low outcome is represented by the failed and lost to follow-up cases. The purpose of the study was to assess the impact of multidrug-resiatnce and MDR-TB on the tuberculosis treatment outcome. Materials and methods. A retrospective selective, descriptive study targeting social, demographic, economic and epidemiological peculiarities, case-management, diagnostic radiological aspects and microbiological characteristics of 187 patients with pulmonary tuberculosis registered during 2013–2015 distributed in two groups: 1st group (61 patients) with established multidrug-resistant strains using conventional cultural methods and the 2nd group (126 patients) with MDR-TB. Results. Multidrug-resistance was established more frequently in new cases and MDR-TB in two thirds of retreated patients. No difference was identified in gender and age distribution, social, economical, educational characteristics; case-management assessment identified a similar proportion of patients revealed by general practitioners and specialists, with low rate of screened high risk groups. All patients from the multidrug-resistant group began the standard treatment for drug-responsiveness tuberculosis before drug susceptibility testing and one third of MDR-TB group was treated from the onset with the DOTS-Plus regimen. Highest success rate was identified in the new-case subgroups of both groups and higher rate of died patients was determined in the retreated subgroups. Such a low rate of patients aggrevates the resistance. Conclusions. Early diagnosis, drug responsiveness testing and raising awareness among about treatment compliance will improve disease outcome.
Introduction
Antituberculosis (anti-TB) drug resistance represents the major problem that threatens the TB control [1, 2]. Usually drug resistance develops due to improper use of chemotherapy (inadequate regimens, patient’s therapeutic incompliance, adverse drug reactions) of drug susceptible TB patiens or due to exogenous infection in regions with high drug resistance burden. Actually drug resistance represents an epidemiological burden in countries with weak TB control programmes [3, 4]. Worldwide 480.000 people developed MDR-TB in 2015. China, India and Russian Federation acount half of the global cases. About 9.5 % of MDR-TB cases had XDR-TB in 2015 [5]. Treatment succes rate of MDR-TB worldwide constituted 52 % and of XDR 28 % in 2015. Republic of Moldova ranks among 30 high multidrug-resistant tuberculosis (MDR-TB) burden countries. The rate of MDR-TB among new Moldovan cases was continuously encreasing (2005 — 13 % till 2014 — 25 %) and in previoulsy treated patients 60 % in the 2012 cohort and 72 % in the 2013 cohort. The treatment succes rate of MDR-TB patients did not exceed 50 % [6].
According to the WHO several drug resistance types are used to establish the adequate case-management and treatment regimen [7]. Mono-resistance is the resistance to one first-line anti-TB drug. Rifampicin resistance is the resistance to rifampicin detected using phenotypic or genotypic methods, with or without resistance to other anti-TB drugs. Poly-resistance is the resistance to more than one first-line anti-TB drug, other than both isoniazid and rifampicin and multidrug-resistance is the resistance to at least both isoniazid and rifampicin. The standard treatment of patients presumed or known to have MDR-TB according to WHO re–commendations in Republic of Moldova is used since 2000 and was extended at the national level in 2005. It consists in a two phase regimen with second-line drugs and lasts 18–24 months. According to the actual national policy the cases with established resistance to rifampicin, or combination of resistance isoniazid-rifampicin, isoniazid-rifampicin-ethambutol, isoniazid-rifampicin-streptomycin are treated in intensive phase during 6–8 months with kanamycin, levofloxacin, ethionamide, cycloserine, pyrazinamide, ethambutol and in continuation phase during 10–16 months with levofloxacin, ethionamide, cycloserine, pyrazinamide and ethambutol [7]. Patients with established resistance to isoniazid-rifampicin-streptomycin-ethambutol are treated with kanamycin, levofloxacin, paraaminosalicylic acid, ethionamide, cicloserine, pyrazinamide during 8 months in intensive phase and levofloxacin, paraaminosalicylic acid, ethionamide, cycloserine-pyrazinamide during 10–16 months in the continuation phase [7]. Clinical monitoring is performed daily if patient is hospitalized and once per week if is treated in ambulatory conditions. In the continuation phase clinical monitoring is performed monthly. Microbiological monitoring (smear microscopy and culture on the conventional methods with second-line drug-susceptibility testing) is performed at the treatment onset, than monthly during the intensive phase and once in three months during the continuation phase. Treatment of MDR-TB costs in average more than US$10.000 per person [2]. Low MDR-TB treatment success rates, significant potential for adverse events, long duration inhibit good treatment compliance and contribute to high rate of therapeutic failure, drop out and death [8]. All related factors associated to high costs contributed to the development of a shorter MDR-TB regimen lasting less than 12 months with a lo–wered costs (< 1.000 $ per patient) [7].
In Republic of Moldova poly-resistant TB is treated according to the results of first-line drug sensitivity testing. The treatment consists of an intensive phase during 2–6 months and continuation phase 4–12 months. Patients with established resistance to isoniazid and/or streptomycin are treated with the combination of rifampicin, pyrazinamide, ethambutol with/without a fluoroquinolone (levofloxacin or moxifloxacin) during 6–9 months. Poly-resistance to isoniazid and ethambutol with/without streptomycine resistance is treated with the combination of rifampicin, fluoroquinolone, ethionamide, aminoglycosides (amikacin, capreomycin or kanamycin) and/or pyrazinamide during 18 months, including 3 months with injectable aminoglycosides (amikacin, capreomycin or kanamycin). Rifampicin monoresistance and polyresistance is treated with the standard MDR-TB regimen. Both poly-resistant and multidrug-resistant tuberculosis show low treatment success rates and high rate of therapeutic failure, drop out and death. The purpose of the study was to assess the impact of poly-resistance and multidrug-resistance on the tuberculosis treatment outcome in the period 2013–2015. Objectives were: 1. Assessment of general, socio-economic and epidemiological risk factors of pulmonary tuberculosis patients with poly-resistance and multidrug-resistance. 2. Evaluation of case-management, diagnosis, radiological aspects and microbiological characteristics of patients with poly-resistant and multidrug-resistant tuberculosis. 3. Establishment the major impact factors on the final treatment outcome of individualised and standard multidrug-resistant tuberculosis regimens.
Materials and methods
It was performed a retrospective selective, descriptive study targeting social, demographic, economic and epidemiological pecualiarities, case-management, diagnosis radiological aspects and microbiological characteristics of 187 patients with pulmonary tuberculosis registered in Chisinau city during 2013–2015. Inclusion criteria were: age > 18 years old, poly-resistance established trough conventional cultural methods in the 1st group (61 patients) and multidrug-resistance established in the 2nd group (126 patients), signed informed consent. Patients from the 1st group were registered in the period 01.01.2013 — 31.12.2015 and the 2nd group in 01.01.2014 — 31.12.2014 in the Municipal Clinical Hospital of Pneumophtysiology of Chisinau city, capital of the Republic of Moldova. The investigational schedule included demographic, social and epidemiological data: sex (male/female ratio), age (distribution in age groups), residence (urban/rural residence, presence of residence card, homeless status), educational level, socio-economic status (employed, unemployed, retired, disabled, student), health and social insurance status, history of migration and detention, presence of high risks (close contact with an infectious source, presence of contacts in the household, comorbiditie), patient’s case-management, treatment cathegory, adverse drug reactions, final outcome. All selected patients were diagnosed and managed according to the National Clinical Protocole 123 “Tuberculosis in adults”. Statistic analysis was carried out using the quantitative and qualitative research methods. Statistical survey was performed –using Microsoft Excel XP soft.
Results and discussion
Distribution in case types according to the WHO definitions established that the 1st group was constituted from two third of new cases comparing with one third of the 2nd group. Retreated cases (relapses, retreated after a previous treatment failure and drop up) were one third of the 1st group and two thirds of the 2nd group. Retreated for a previous standard drug-susceptible treatment failure were more frequently patients in the 2nd group comparing with the 1st group. Relapses were previously treated patients, declared cured or treatment completed, than diagnosed with a recurrent episode of tuberculosis. Treatment failure was considered the patients established microbiological positive at the end of 5th month. Loss to follow-up were patients that interrupted the treatment for more than 2 months (table 1).
Smear microscopy identified a similar rate o microscopic positive for acid-fast-bacilli patients in both groups. Culture positive were the majority of patients due to the inclusion criteria of available established drug resistance. Conventional phenotype drug sensitivity testing on Lowenstein-Jensen medium and BACTEC identified following first-line drug resistance: in the 1st group 48 (78.69 %) were resistant to HS, 10 (16.39 %) to HES and 3 (4.91 %) to HE; in the 2nd group 90 (71.43 %) were resistant to HRSE, 33 (26.19 %) to HRS, 3 (2.38 %) to HRE. Among all MDR-TB patients 17 (13.49 %) patients were resistant to ethionamide and 3 (4.35 %) were resistant to levofloxacin and kanamycin, defined as extensively drug resistant TB (XDR-TB). It is important to note a higher rate of available results for 1st line drug-susceptibility testing in the first three months after the onset of the treatment in the 1st group comparing with the 2nd group, due to the established role of positive and resistant result at the molecular genetic test GeneXpert MTB/Rifampicin in the diagnosis of rifampicin and/or MDR-TB. For 2nd line-susceptibility were tested each third patient from the 1st group and each fifth patient from the 2nd group. GeneXpert MTB/Rifampicin positive was positive in a similar rate in both groups and resistant to rifampicin were identified only in the 2nd group due to specific inclusion criteria, that permitted to diagnose multidrug-resistance (table 2).
Sex distribution in the 1st group identified a male-female ratio 5.1/1 with 51 (83.61 %) men and 10 (16.39 %) women comparing with the 2nd group with male-female ratio 3.34/1 with 97 (76.98 %) men and 29 (23.02 %). No difference was identified regarding the distribution of patients in age groups. However was identified the predominance of the 35–54 age group in both samples, following by the 18–34 years group. Distribution of patients accor–ding to the demographic characteristics identified that lack of residence card (visa) or homeless status was established in the each fifth patient of both groups. So, distributing patients according to the biological characteristics it was argued that men and young age individuals have the same probability to have any drug resistance. Demographic distribution identified that the majority of patients were from urban areas. It was established that one tenth patient had homeless status (table 3).
/20-1.jpg)
Distributing patients according to the economic status, it was established that employed persons were in a low rate in both group and unemployed patients were one half of both groups. Disease disabled were only every tenth patient, despite the fact that the national policies permit multidrug-resistant patient to get tuberculosis-related financial. One half of both groups had no health and social protection due to no contributing to the health budget by paying taxes, health insurance policy and social taxes (table 4).
Assessing the educational level it was established that the most of the patients from both groups graduated general school or lyceum. Incomplete general school slightly predominated in the 1st group. Other educational levels were similar distributed among groups (table 5).
Distributing patients in high risk groups established that previous anti-TB treatment showed the biggest impact on the developing MDR-TB and co-morbidities on the expansion of poly-resistance. History of migration in the last 12 months, history of detention and contact with an infectious source slightly predominated in the 2nd group and alcohol abuse in the 1st group. So, the distribution of drug resistant patients established the primary target groups in frame of which must be performed an adequate drug sensitivity testing are patients included in retreatment regimens and patients with co-morbidities (table 6).
/21-1.jpg)
Studying case-management it was identified that general practitioners detected 44 (72.14 %) patients of the 1st group comparing with 72 (57.14 %) patients of the 2nd group. High risk group screening was used in a similar proportion to detect patients from both groups 14 (22.95 %) in the 1st group and 26 (20.62 %) in the 2nd group. Direct addressing to the specialized clinical services was more frequently used by the patients of the 2nd group due to higher proportion of those included in retreatment regimen (table 7).
All patients from the 1st group were treated starting with the onset till the availability of culture drug susceptibi–lity testing with the standard regimen for established/presumed drug susceptible TB, than in 51 (83.6 %) cases was replaced with individualized regimen according to the drug-resistance profile. Patients from the 2nd group were treated starting with the onset till the availability of conventional culture drug sensitivity testing with: 1st line drugs according to the standard regimen for susceptible tuberculosis — 60 (47.62 %) cases, individualized regimens were used for 11 (8.73 %) cases and with standard DOTS-plus for MDR-TB were treated 46 (36.51 %) cases.
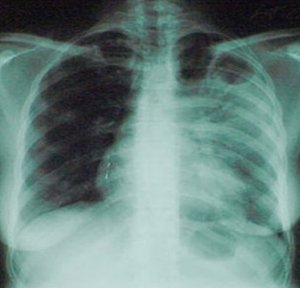
Identifying the clinical radiological forms of pulmonary tuberculosis it was established that pulmonary infiltrative tuberculosis was diagnosed in the most of patients from both groups. Other radiological forms such as disseminated tuberculosis slightly predominated in the 1st group and fibro-cavernous tuberculosis in the 2nd group. Distributing patients according to the number of the affected lungs it was established that one lung was involved in two third of the 2nd group and both lungs were affected in two third of the 1st group. Infiltrative opacities and destructive forms of pulmonary tuberculosis were identified in a similar proportion of both groups, but extensive forms of pulmonary tuberculosis predominated in the 1st group. It can be explained by the fact the molecular genetic test GeneXpert MTB/Rif contributed to an early detection of the patients from the 2nd group with more localized and less severe forms of pulmonary tuberculosis than those from the 1st group (table 8).
/22-1.jpg)
Distributing patients according to the case-type it was established that new cases statistically predominated in the 1st group and patients included in retreatment regimen were more frequently in the 2nd group. Stratifying patients according to the outcome it was established higher success rate in the new-case subgroups from both drug resistant groups and higher rate of died patients in the retreated subgroups. It is important to note that 4 (6.56 %) from the 1st group enhanced the resistance till MDR-TB and 3 (6.12 %) from the 2nd group developed XDR-TB. Lost to follow-up were more frequently patients from the 1st group (9 (14.75 %) vs. 8 (6.35 %) cases of the 2nd group) and were continuing the treatment more frequently patients form the 2nd group ((26.98 %) vs. 6 (9.84 %) patients from the 1st group).
Conclusions
Poly-resistant TB is established more frequently in new cases comparing with multidrug-resistance identified in two third of retreated patients.
Sex and age distribution established similarity of both groups of drug resistant patients.
No differences were identified regarding distribution according to the social, economical and educational characteristics.
Case-management assessment identified a similar proportion of patients detected by general practitioners and specialists, with a low rate of the screened high risk groups. Direct addressing to the specialized clinical services was more frequently used by the MDR-TB patients due to higher proportion of those included in retreatment regimen.
All poly-resistant patients were treated starting with the onset till the availability of drug susceptibility tes–ting with the standard regimen for established/presumed drug susceptible TB, and one third of MDR-TB group was treated from the onset with the standard DOTS-plus regimen.
Infiltrative opacities and destructive forms of pulmonary tuberculosis were identified in a similar proportion of both groups, but extensive forms of pulmonary tuberculosis predominated in the poly-resistant group.
Highest success rate was identified in the new case subgroup of the poly-resistant group and highest rate of died patients was established in the retreated subgroup of MDR-TB. A similar low rate of patients from both groups enhanced the resistance.
Primary target groups in frame of which must be performed an adequate drug susceptibility testing and early adequate treatment represent patient with previous anti-TB treatment and patients with co-morbidities.
Early diagnosis, adequate drug susceptibility testing and raising awareness among TB patients about treatment compliance will improve disease outcome.
Conflicts of interests. Authors declare the absence of any conflicts of interests that might be construed to in–fluence the results or interpretation of their manuscript.
Authors participation:
Study concept and design: Lesnic E., Todoriko L.
Collection and processing of the material: Lesnic E., Niguleanu A., Ustian A.
Statistical processing of data: Lesnic E., Todoriko L.
Text: Lesnic E., Todoriko L.

/20-1.jpg)
/21-1.jpg)
/22-1.jpg)
/23-1.jpg)