Журнал «Здоровье ребенка» Том 14, №2, 2019
Вернуться к номеру
Випадок стронгілоїдозу, поєднаного з сальмонельозом, у немовляти: міркування з діагностики
Авторы: V.V. Mavrutenkov(1), A.V. Cherginets(1), O.V. Shvaratska(1), L.M. Cherginets(2)
(1) — State Institution “Dnipropetrovsk Medical Academy of the Ministry of Health of Ukraine”, Dnipro, Ukraine
(2) — City Clinical Children’s Hospital 6, Dnipro, Ukraine
Рубрики: Педиатрия/Неонатология
Разделы: Справочник специалиста
Версия для печати
Актуальність. Поєднані інфекції в педіатрії є клінічною проблемою, що набуває актуальності через їх поширеність і тенденцію до зміни типової клінічної картини окремих захворювань. Відповідно, це ускладнює точну оцінку етіології, процес лікування та негативно впливає на результат. Враховуючи зміни клімату, значні міграційні потоки та міжнародний туризм, тропічні гельмінози, які раніше не були поширені в Україні, є реальною загрозою для здоров’я населення, особливо в поєднанні з іншими патогенами. Матеріали та методи. Ми спостерігали випадок стронгілоїдозу та сальмонельозу в 5-місячної дитини, яка не відвідувала будь-якої суб- або тропічної території земної кулі. Дівчина походить із соціально незахищених верств суспільства і була залишена безпритульними батьками відразу після госпіталізації. У дівчини відмічались виражені тяжкі токсичні прояви, діарея, затримка розвитку, порушення харчування та зневоднення від помірного до тяжкого ступеня, плямисто-папульозні висипання на тулубі й нижніх кінцівках. Пряма світлова мікроскопія фекалій виявила Strongyloides stercoralis у кількості понад 10 рухливих личинок у полі зору, на різних стадіях розвитку. Промивна рідина бронхіального лаважу не містила личинок Str. stercoralis. Фекальна культура виявила Salmonella enteritidis групи D. Хіміотерапія цефтріаксоном внутрішньовенно й альбендазолом перорально призвела до елімінації обох патогенів. Висновки. Цей випадок змішаної інфекції S. enteritidis і Str. stercoralis може розглядатися лише як імовірний випадок автохтонної інфекції Str. stercoralis, оскільки вона не була підтверджена більш надійними методами діагностики (наприклад, ПЛР-визначення ДНК Str. stercoralis) і демонструє сумнівну епідеміологічну історію. Отже, для поліпшення діагностики ендемічних паразитарних інфекцій необхідно запровадити таку верифікацію як обов’язкову та обов’язково реєструвати відповідні випадки в національній системі епідеміологічного нагляду та біологічної безпеки.
Актуальность. Сочетанные инфекции в педиатрии являются клинической проблемой, приобретающей актуальность из-за их распространенности и тенденции к изменению типичной клинической картины отдельных заболеваний. Соответственно, это затрудняет точную оценку этиологии, процесс лечения и негативно влияет на результат. Учитывая изменения климата, значительные миграционные потоки и международный туризм, тропические гельминтозы, которые ранее не были распространены в Украине, являются реальной угрозой для здоровья населения, особенно в сочетании с другими патогенами. Материалы и методы. Мы наблюдали случай стронгилоидоза и сальмонеллеза у 5-месячного ребенка, который не посещал никаких суб- или тропических территорий земного шара. Ребенок происходит из социально незащищенных слоев общества и оставлен бездомными родителями сразу после госпитализации. У девочки отмечались выраженные тяжелые токсические проявления, диарея, задержка развития, нарушение питания и обезвоживание от умеренной до тяжелой степени, пятнисто-папулезные высыпания на туловище и нижних конечностях. Прямая световая микроскопия фекалий обнаружила Strongyloides stercoralis в количестве более 10 подвижных личинок в поле зрения, на разных стадиях развития. Промывные воды бронхиального лаважа не содержали личинок Str. stercoralis. Фекальная культура обнаружила Salmonella enteritidis группы D. Химиотерапия цефтриаксоном внутривенно и альбендазолом перорально привела к элиминации обоих патогенов. Выводы. Данный случай смешанной инфекции S. enteritidis и Str. stercoralis может рассматриваться только как вероятный случай аутохтонной инфекции Str. stercoralis, поскольку она не была подтверждена более надежными методами диагностики (например, ПЦР-определение ДНК Str. stercoralis) и демонстрирует сомнительную эпидемиологическую историю. Соответственно, для улучшения диагностики эндемичных паразитарных инфекций необходимо ввести такую верификацию как обязательную и обязательно регистрировать соответствующие случаи в национальной системе эпидемиологического надзора и биологической безопасности.
Background. Pediatric co-infections are an emerging clinical problem due to their increasing prevalence and tendency to transform a typical clinical presentation of particular diseases. Thereafter, it tangles the accurate estimation of etiology, complicates the management and negatively impacts the outcome. Given the climatic changes, significant migratory flows and international tourism, tropical helminthiases, previously not common in Ukraine, are a real threat to the public health, especially in combination with other pathogens. Materials and methods. We observed a case of strongyloidiasis and salmonellosis in a Ukrainian 5-month-old female infant who had no history of visiting any of the sub- or tropical territory of the globe. The girl came from a socially vulnerable family and was abandoned by her homeless parents immediately after admission. The girl presented with severe toxic manifestations, diarrhea, developmental delay, moderate-to-severe malnutrition and dehydration, and maculopapular rash on the trunk and lower extremities. Direct light microscopy of feces revealed Str. stercoralis in the number of more than 10 mobile larvae per high-power field, at different stages of development. Bronchial lavage fluid contained no larvae of Str. stercoralis. Fecal culture revealed group D S. enteritidis. Chemotherapy with ceftriaxone IV and oral albendazole resulted in elimination of both pathogens. Conclusions. The given case of S. enteritidis and Str. stercoralis co-infection should be considered as a probable case of autochthonous Str. stercoralis infection, as it was not confirmed by more reliable diagnostic methods (e.g. PCR for Str. stercoralis DNA), and demonstrates a doubtful epidemiological history. Consequently, to improve the diagnosis of endemic parasitic infections, it is necessary to introduce such a verification as compulsory, and mandatory registration of relevant cases in the Ukrainian National System of Epidemiological Surveillance and Biosecurity is required.
стронгілоїдоз; сальмонельоз; мікст-інфекція; діагностика; діти
стронгилоидоз; сальмонеллез; микст-инфекция; диагностика; дети
strongyloidiasis; salmonellosis; co-infection; diagnosis; children
Introduction
Pediatric co-infections, especially in infants, are an emerging scientific and practical issue of concern due to the clinical nosomorphosis (a transformation of the typical signs of a particular disease), which tangles the accurate estimation of etiology, impedes the management and negatively impacts the outcome.
The WHO estimated that about 30 % of all deaths caused by intestinal infections are registered among children aged five years and younger, despite the fact that they account for only 9 % of the world’s population. In these circumstances, non-typhoid Salmonella serotypes are among the top three etiologically significant agents of acute diarrhea, being responsible for about 2000 deaths in Europe every year [1].
According to various sources, epidemiological cumulative data on the prevalence of strongyloidiasis worldwide vary ranging from 3 million to 100 million, indicating the global spread of the helminthiasis [2, 3]. Autochthonous cases of strongyloidiasis in Europe were registered in the Mediterranean region, especially in Spain and Italy. There are sporadic reports on the presence of foci of strongyloidiasis in other regions of the Europe. Currently, strongyloidiasis in Europe is recorded mainly in immigrants or travelers attending the endemic areas [4–7]. In Ukraine, autochthonous foci of strongyloidiasis were identified by epidemiological studies in the south-western part of Ukraine in Odesa region [8].
Considering climate change in the form of global warming, large migration flows and international tourism, tropical helminthiases, especially in case of combination with other pathogens, are a real threat to the public health while the Ukrainian healthcare system is being fundamentally reformed nowadays.
Thereby, our paper is aiming to improve clinical diagnosis and management of endemic parasitic infections by discussing a clinical case of a presumed strongyloidiasis in an infant.
Materials and methods
The paper describes a verified case of strongyloidiasis mixed with Salmonella infection in a 5-month-old female infant who has never visited any of the sub- or tropical territories of the globe.
Case presentation
A girl M., 5 months old, ethnic Romany, was brought along with her mother to one of the children’s hospitals of Dnipro by police officers in early August 2018. The mother was homeless, in the last few weeks she had been residing with her child at the railway station of Kamianske (Dnipropetrovsk region). According to the mother, she and her child had recently arrived from the city of Berehove (Zakarpattia region) and never stayed outside Ukraine. In order to feed the child, the woman used any food found in the street trash cans or provided by strangers.
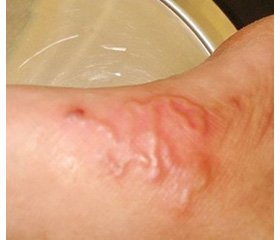
On admission, the child was ill-appearing and presented with failure to thrive, fever up to 39.5° С, decreased nutritional dominant and diarrhea. An examination revealed dystrophic cutaneous changes and maculopapular rash on the lower abdomen, anogenital zone, and lower extremities, as well as grayish skin tone and poor skin turgor (skin turgor test ≥ 5 seconds); the capillary refill time was 2 seconds. The body mass index was beyond 3 standard deviations below the mean. When performing assessment of the child’s development in accordance with the current Order of the Ministry of Health of Ukraine No. 149 dated March 20, 2008 “On approval of the Clinical Protocol for the Medical Surveillance of a Healthy Child under 3 Years of Age”, we found developmental delay, general muscle hypotonia, poor reaction to the external stimuli, and weak cry. The level of consciousness assessed by the Pediatric Glasgow Coma Scale was estimated at the total score of 14 points (minus 1 point: verbal response to irritation). We didn’t observe any organic neurological deficiency. The anterior fontanel was soft but modestly sunken (the child’s neurological status was assessed in accordance with the MoH of Ukraine Order No. 437 dated August 31, 2004 “Protocols for Medical Care in Medical Emergencies in Children at the Hospital and Pre-hospital Stages”). Upon the examination of the cardiovascular and respiratory system, the child presented with compensatory tachypnea and tachycardia corresponding to the grade of fever, with normal saturation and thus without any necessity for artificial cardiorespiratory support. No cough was observed. Chest X-ray examination showed no inflammatory or destructive changes or anomalies, but the II degree thymomegaly. Abdominal examination revealed flatulence and moderate sensitivity to palpation, accelerated bowel sounds on auscultation. Abdominal ultrasonography showed normal kidney structure and moderate hepatosplenomegaly, however, no organic lesions or anomalies were found in either of organs. The child had up to ten stools per day, classified as type 7 on the modified pediatric Bristol Stool Chart [9].
On admission, complete blood count revealed moderate leukocytosis of myeloid type with 3 % of eosinophils and ESR 28 mm/h. Serum C-reactive protein accounted for 16 mg/ml. We observed moderate depletion of serum electrolytes and preserved acid-base balance as following: sodium 129 mmol/l, potassium 3.1 mmol/l, chlorides 92 mmol/l, and bicarbonate 20 mmol/l. The blood glucose level and the serum content of the main biochemical constants reflecting liver and kidney function were within normal values. Coprocytogram showed 60 white blood cells per HPF, and considerable amount of neutral fat. Direct light microscopy of feces revealed Strongyloides stercoralis in the quantity of more than 10 motile larvae per field, at different stages of development. Bronchoalveolar lavage fluid analysis with direct light microscopy identified no Str. stercoralis larvae. Conventional fecal culture revealed group D Salmonella enteritidis. ELISA test for serum HIV antibodies was negative.
Given the results of clinical, epidemiological, laboratory and instrumental studies, a clinical diagnosis was as following. Mixed infection: strongyloidiasis, intestinal form in combination with Salmonellosis (S. enteritidis), enteric form, moderate severity. Complications: second degree isotonic dehydration. Concomitant disease: second degree alimentary malnutrition.
The treatment program included: 1) calculation of daily calories based on the actual body weight and compensation for energy requirements by inclusion of an adapted infant formula (due to poor nutritional behaviour and high risk of aspiration, tube feeding were applied during the first days of treatment, and further, with the child’s improvement, it was replaced with oral food and fluid intake); 2) crystalloid IV solutions considering electrolyte deficit and acid-base balance; 3) systemic antibiotic chemotherapy (ceftriaxone IV 50 mg/kg/day within seven days); 4) systemic antiparasitic chemotherapy (oral albendazole at a dose of 15 mg/kg/day in two divided doses within ten days). We performed antiparasitic chemotherapy control by repeated microscopy of feces: on the fifth day of treatment Str. stercoralis larvae in the feces lost their motility, on the seventh and nineth days of therapy they were not found.
After completion of albendazole course, we performed parasitological studies of feces for the presence of Str. stercoralis larvae every three days. Two weeks after completion of systemic antiparasitic chemotherapy, despite the absence of Str. stercoralis larvae in feces, a repeated three-day course of albendazole at a dose of 15 mg/kg/day in two divided doses was administered aiming to prevent autoreinvasion. During treatment and subsequently at the rehabilitation stage the child did not experience any adverse reactions to the systemic anti-infectious chemotherapy.
After successful treatment of the acute phase of the disease, the therapeutic focus was shifted to rational nutrition with a daily calculation of calorie needs and ingredients, and physical methods of rehabilitation. During the following month, the child demonstrated a sustainable body weight increment and a gradual recovery of developmental progression and emotional tone. As the mother did not visit the child in the pediatric clinic and her location was unknown, the child was transferred by the child protection authorities to a specialized childcare setting. At present, the child is clinically healthy, thriving and developing gradually. Control parasitological fecal testing for Str. stercoralis and group D S. enteritidis was negative.
Discussion
Two interrelated issues form the special trait of the case presented: firstly, where an infant who had not been on the endemic territories could get infected with strongyloidiasis, and, secondly, under what conditions a child with substantial risk factors for the adverse course of the disease (i.e. protein energy malnutrition and dehydration), having a mixed infection of strongyloidiasis and group D S. enteritidis which is a serious pathogen, fortunately, was able to recover and completely sanitize against the infection.
According to the definition, strongyloidiasis is an anthroponotic geohelminth infection caused by Strongyloides stercoralis and typical for the subtropical and tropical regions of the globe, where the free-living transitional forms of the pathogen (filariform larvae) are found in soil.
If we turn to the epidemiology of strongyloidiasis, it should be noted that the source of invasion is a person who spreads rhabditiform larvae of the helminth with feces. In soil, under favorable conditions, these larvae molt four times and form a free-living generation which produces eggs giving a new generation of free-living rhabditiform larvae, or infective filariform larvae (the indirect route of development). Under adverse environmental conditions, particularly in temperate climates, rhabditiform larvae transform into infective filariform larvae in 12–48 hours (the direct route of development). The latter can be formed directly in the small intestine, causing autoinvasion [10]. Human infection results from the filariform larvae percutaneous penetration or oral transmission, but in the latter case, the filariform larvae actively penetrate the mucous membranes of the oral cavity and esophagus. The Fig. 1 demonstrates strongyloid’s life cycle [11].
The parasitic helminth Str. stercoralis is endemic for subtropical and tropical regions where the number of people infected reaches 100 million, but can occur in the Southern and Eastern regions of Europe (Table 1) [12].
Thus, we can expect the distribution of geohelminth Str. stercoralis infection in the southern regions of Ukraine, which is being confirmed by epidemiological studies by T.Ya. Pogorelchuk (2007). Thus, according to the results of these studies in Odesa region, Str. stercoralis larvae at different stages of development were found in soil, on the surface of vegetables and tools (shovels, rakes, etc.) and agriculture workers’ clothes. Also, 147 adults with strongyloidiasis were identified [8]. We do not know how the child was travelling, but she was born in Zakarpattia region. Given that the incubation period of strongyloidiasis does not exceed a month [13], and the child was admitted in the early autumn, the probability of autochthonous origin of the helminth cannot be ruled out. Medical examination of the mother with collection of the epidemiological history could have been an important measure in confirming the autochthonous origin of Str. stercoralis in the infant, yet, as already mentioned above, the woman abandoned the child and resolutely refused to be admitted, and then never attended her daughter. Thus, the source of the child’s infection remained unclear and, from the epidemiological point of view, the diagnosis of strongyloidiasis remains unverified.
The second disputable point is the child’s recovery and clearance from infection, considering that numerous predictors of an adverse course of the disease were present, such as: mixed infection with S. enteritidis, which predisposes to septic course; early childhood; protein energy malnutrition; dehydration; parental care deprivation. It should be emphasized that in terms of immune incompetence, which is present in infants with protein energy malnutrition, the number of parasites can be pretty significant. This leads to extraintestinal allocation of helminthes with multiple organ lesions that cause severe, fatal or chronic (autoinvasion) strongyloidiasis course [14–16]. Moreover, albendazole used for antiparasitic chemotherapy does not apply to the first choice drug list due to significantly lower efficacy in comparison with ivermectin, which is the first-line treatment for strongyloidiasis [17–19]. Likewise, in case of autoinvasion, which should have been expected in the child, a conventional short-term course of albendazole would likely have not been completely effective.
Given the above, the question arises, whether exactly Str. stercoralis was found in the child. And if it was not Str. stercoralis, then what could have it been? According to laboratory tests performed in a child, the diagnosis of strongyloidiasis was justified merely by the presence of Str. stercoralis larvae in feces, which might be precarious (Table 2) [13, 20, 21].
To clarify what precisely could have been found when investigating the feces by direct light microscopy (helminthoscopy), we referred to the microbiology of nematodes of the Rhabditida order, Strongyloididae family, Strongyloides genus. We ascertained that obligate zoonotic strongyloidiasis of sheep, goats, cattle, horses and other animals is widely distributed on the territory of Dnipropetrovsk and Poltava regions [22–25]. Given this, we hypothesize that the child could swallow zoonotic helminths of the Strongyloides genus, which passed the intestine without causing injury, because the physiology of zoonotic forms coheres with the physiology of animals, which is certainly pretty different from the human one. Moderate transient blood eosinophilia in the child which occurred only after albendazole administration may be considered as the endorsement of the presumed intestinal passage of zoonotic helminths of the Strongyloides genus (Table 3). While elevated levels of leukocytes and especially eosinophils in the peripheral blood are a concomitant attribute of migratory forms of nematodes (larvae), as well as of chronic (intestinal) forms of strongyloidiasis [2, 26, 27].
Considering the above mentioned, we can assume that in the case discussed there might be a laboratory error while performing fecal microscopy. Thus, it should be emphasized that for the verification of endemic infections it is necessary to apply the full range of investigations with the use of modern immunochemistry methods as well as to cooperate with the veterinary epizootic control service. Also, in our opinion, for improving the level of control and to counteract the spread of endemic parasitic infections, a revision of the Order of the Ministry of Health of Ukraine No. 905 dated December 28, 2015 “On Approval of the Criteria for Determining the Cases of Infectious and Parasitic Diseases to be Registered” is necessary, with inclusion of Str. stercoralis infection to the list.
Conclusions
1. The presented clinical case of mixed infection of salmonellosis and strongyloidiasis in relation to the latter should be considered only as a presumed case of autochthonous Str. stercoralis infection, given the lack of justification with secure laboratory methods and epidemiological history.
2. In order to improve the diagnosis of endemic parasitic infections, it is necessary to introduce mandatory verification in reference laboratory settings by reliable laboratory tests, which are based on immunochemistry methods and molecular-genetic analysis.
3. A revision of the legislative and regulatory base for parasitic diseases is required to put Str. stercoralis infection in the list of parasitic diseases that are subject to mandatory registration in the National System of Epidemiological Surveillance and Biological Safety of the public health system of Ukraine.
Conflicts of interests. Authors declare no conflicts of interests that might be construed to influence the results or interpretation of their manuscript.
Authors’ contributions: Victor Mavrutenkov — idea and design of the article, draft manuscript preparation, revision of the manuscript; Artyom Cherginets — data collection and analysis, draft manuscript preparation; Olha Shvaratska — data analysis, draft manuscript preparation, final editing of the manuscript, English version of the manuscript; Lina Cherginets — data collection and analysis.
1. Davies O.L. WHO’s first ever global estimates of foodborne diseases find children under 5 account for almost one third of deaths [Electronic source] / O.L. Davies, F. Chaib // WHO News Release, Geneva. — 2015. — doi: who.int/news-room/detail/03-12-2015-who-s-first-ever-global-estimates-of-foodborne-diseases-find-children-under-5-account-for-almost-one-third-of-deaths.
2. Siddiqui A.A. Strongyloidiasis / A.A. Siddiqui, R.M. Genta, S.L. Berk // Tropical Infectious Diseases: Principles, Pathogens and Practice: 2nd ed. — Philadelphia: Churchill Livingstone (Elsevier), 2005. — Vol. 1. — P. 1274-1285.
3. Bisoffi Z. Strongyloides stercoralis: a plea for action / Z. Bisoffi, D. Buonfrate, A. Montresor et al. // PLoS neglected tropical diseases. — 2013. — Vol. 7, Iss. 5. — P. e2214. doi: 10.1371/journal.pntd.0002214.
4. Schär F. Strongyloides stercoralis: Global Distribution and Risk Factors / F. Schär, U. Trostdorf, F. Giardina et al. // PLoS neglected tropical diseases. — 2013. — Vol. 7, Iss. 7. — P. e2288. doi: 10.1371/journal.pntd.0002288.
5. Valerio L. Strongyloides stercoralis, the hidden worm. Epidemiological and clinical characteristics of 70 cases diagnosed in the North Metropolitan Area of Barcelona, Spain, 2003–2012 / L. Valerio, S. Roure, G. Fernández-Rivas et al. // Transactions of the Royal Society of Tropical Medicine and Hygiene. — 2013. — Vol. 107, Iss. 8. — P. 465-470. doi: 10.1093/trstmh/trt053.
6. Buonfrate D. Epidemiology of Strongyloides stercoralis in northern Italy: results of a multicentre case-control study, February 2013 to July 2014 / D. Buonfrate, M. Baldissera, F. Abrescia et al. // Eurosurveillance. — 2016. — Vol. 21, Iss. 31. — P. 30310. doi: 10.2807/1560-7917.ES.2016.21.31.30310.
7. Поляков В.Е. Стронгилоидоз у детей / В.Е. Поляков, И.А. Иванова, Н.Р. Полякова, М.Л. Воробьевa, Н.В. Поляков, В.В. Ромих // Педиатрия. — 2015. — Т. 94, № 5. — C. 120-125.
8. Погорельчук Т.Я. Особливості розповсюдження і клінічних проявів стронгілоїдозу у жителів Одеської області: автореф. дис. на здобуття наук. ступеня канд. мед. наук: спец. 16.00.11 «Паразитологія». — К., 2007. — 24 с.
9. Nutrition Guideline Healthy Infants and Young Children GI Function: Management of Constipation / Alberta Health Services. — Canada, 2015. — 14 p. — Режим доступу: https://www.albertahealthservices.ca/assets/info/nutrition/if-nfs-ng-healthy-infants-gi-function-management-constipation.pdf.
10. Page W. The Unique Life Cycle of Strongyloides stercoralis and Implications for Public Health Action / W. Page, J.A. Judd, R.S. Bradbury // Tropical medicine and infectious disease. — 2018. — Vol. 3, Iss. 2. — P. 53. doi: 10.3390/tropicalmed3020053.
11. The Free Dictionary by Farlex. Medical Dictionary: Strongyloides: Life cycle of Strongyloides stercoralis (from Mahon and Manuselis, 2000) [Electronic source]. — Режим доступу: https://medical-dictionary.thefreedictionary.com/Strongyloides.
12. Farthing M. WGO Practice Guideline: Management of Strongyloidiasis / M. Farthing, S. Fedail, L. Savioli, D.A.P. Bundy, J.H. Krabshuis // WGO. — 2004. — Режим доступу: http://www.worldgastroenterology.org/guidelines/global-guidelines/management-of-strongyloidiasis.
13. Farrar J. Manson’s Tropical Diseases / J. Farrar, P. Hotez, T. Junghanss, G. Kang, D. Lalloo, N. White. — 23rd edition. — Elsevier: Saunders LTD, 2013. — P. 1024.
14. Yap P. Determining soil-transmitted helminth infection status and physical fitness of school-aged children / P. Yap, T. Fürst, I. Müller, S. Kriemler, J. Utzinger, P. Steinmann // Journal of visualized experiments. — 2012. — Iss. 66. — P. e3966. doi: 10.3791/3966.
15. Long S.S. Principles and Practice of Pediatric Infectious Diseases / S.S. Long, L. Pickering, C. Prober. — 3th ed. — Stanford: Churchill Livingstone Elsevier, 2008. — 1684 p.
16. Nutman T.B. Human infection with Strongyloides stercoralis and other related Strongyloides species / T.B. Nutman // Parasitology. — 2016. — Vol. 144, Iss. 3. — P. 263-273. doi: 10.1017/S0031182016000834.
17. Bradley J.S. Nelson’s Pediatric antimicrobial therapy / J.S. Bradley, J.D. Nelson. — 22nd ed. — New York: Amer. Acad. Pediatrics, 2016. — 278 p.
18. Krolewiecki A.J. A public health response against Strongyloides stercoralis: time to look at soil-transmitted helminthiasis in full / A.J. Krolewiecki, P. Lammie, J. Jacobson et al. // PLoS neglected tropical diseases. — 2013. — Vol. 7, Iss. 5. — P. e2165. doi: 10.1371/journal.pntd.0002165.
19. Forrer A. Ivermectin Treatment and Sanitation Effectively Reduce Strongyloides stercoralis Infection Risk in Rural Communities in Cambodia / A. Forrer, V. Khieu, C. Schindler et al. // PLoS neglected tropical diseases. — 2016. — Vol. 10, Iss. 8. — P. e0004909. doi: 10.1371/journal.pntd.0004909.
20. Токмалаев А.К. Стронгилоидоз / А.К. Токмалаев, Г.М. Кожевникова // Клиническая паразитология: протозоозы и гельминтозы. — М.: МИА, 2017. — 385 c.
21. Amor A. High prevalence of Strongyloides stercoralis in school-aged children in a rural highland of north-western Ethiopia: the role of intensive diagnostic work-up / A. Amor, E. Rodriguez, J. Saugar et al. // Parasites & vectors. — 2016. — Vol. 9, Iss. 1. — P. 617. doi: 10.1186/s13071-016-1912-8.
22. Гугосьян Ю.А. Стронгілоїдоз коней (поширення, діагностика, заходи боротьби): автореф. дис. на здобуття наук. ступеня канд. вет. наук: спец. 16.00.11 «Паразитологія» / Гугосьян Юрій Андрійович; Львівський національний університет ветеринарної медицини та біотехнологій імені С.З. Ґжицького. — Львів, 2018. — 24 с.
23. Бойко О.О. Порівняння двох методів підрахунку яєць нематод / О.О. Бойко, Ю.В. Дуда // Сучасні тенденції проведення лабораторних досліджень у ветеринарній медицині: Мат-ли Всеукраїнського наукового семінару, присвяченого 20-річчю заснування кафедри паразитології та ветеринарно-санітарної експертизи Полтавської державної аграрної академії. Полтава, 19 травня 2015 року. — Полтава: ТОВ НВП «Укрпромторгсервіс», 2015. — С. 23.
24. Мельничук В.В. Епізоотологічна ситуація щодо інвазійних захворювань свиней на території Полтавської області / В.В. Мельничук, В.О. Пругло // Сучасні тенденції проведення лабораторних досліджень у ветеринарній медицині: Мат-ли Всеукраїнського наукового семінару, присвяченого 20-річчю заснування кафедри паразитології та ветеринарно-санітарної експертизи Полтавської державної аграрної академії. Полтава, 19 травня 2015 року. — Полтава: ТОВ НВП «Укрпромторгсервіс», 2015. — С. 66.
25. Шендрик І.М. Виявлення здатності мікобактерій до персистування в організмі личинок Strongуloides papillosus за умов їх сумісного культивування / І.М. Шендрик // Сучасні тенденції проведення лабораторних досліджень у ветеринарній медицині: Мат-ли Всеукраїнського наукового семінару, присвяченого 20-річчю заснування кафедри паразитології та ветеринарно-санітарної експертизи Полтавської державної аграрної академії. Полтава, 19 травня 2015 року. — Полтава: ТОВ НВП «Укрпромторгсервіс», 2015. — С. 96.
26. Дж.Д. Клейн и др. Секреты лечения детских инфекций / Под ред. В.Ф. Учайкина. — М.: Бином, 2007. — 415 с.
27. Toledo R. Strongyloidiasis with emphasis on human infections and its different clinical forms / R. Toledo, C. Muñoz-Antoli, J.G. Esteban // Advances in Parasitology. — 2015. — Vol. 88. — P. 165-241. doi: 10.1016/bs.apar.2015.02.005.

/113-1.jpg)
/114-1.jpg)
/115-1.jpg)