Introduction
Scarlet fever is an acute anthroponotic infectious disease characterized by exudative pharyngitis, fever, and bright red exanthem. It is caused by streptococcal pyrogenic exotoxins types A, B, and C produced by group A beta-hemolytic streptococci, which can be found in secretions and discharge from the skin, nose, throat, and ears. Scarlet fever is associated with purulent-septic and allergic complications [1–5]. Toxicodermia is an inflammation of the skin caused by any toxic substance [10, 11]. Therefore, both diseases can be combined in one clinical case.
The etiology of toxicodermia combined with scarlet fever in children is complex and not fully studied, and probably potentiated by toxins synthesized by group A beta-hemolytic streptococci. Due to the severity of skin reactions, early detection, diagnosis, treatment, and the development of preventive agents in scarlet fever with toxicodermia are important for improving clinical outcomes.
Case presentation
A girl aged 12 years was hospitalized to Ternopil Regional Children’s Clinical Hospital with complaints of the rise in temperature to 37.9 °C, headache, sore throat, and painful swallowing, ophthalmalgia, hyperemia of the skin and a characteristic finely spotted rash on the face, trunk, and extremities.
Morphologically, rashes are finely speckled, finely spotted elements with a tendency to merge, spread by area; they form a dense pink field on the skin. The disease began acutely one day before. During 2–3 days, the rashes spread over the body with the predominant localization on the face, trunk, extremities and appeared on the oral mucosa. According to the history of the disease, it is known that before the onset of symptoms, the girl played with a synthetic toy made of polymeric materials. Also, according to parents, contact redness of hands and itching of the skin were noted previously. Two weeks before the disease, they visited the otolaryngologist for the sore throat. Antibiotic therapy was not prescribed, only symptomatic treatment.
Objective examination. The general condition of the child is of moderate severity due to the exanthem and intoxication syndromes. Weight 54 kg, height 160 cm. The girl is conscious, reacts to the examination adequately. Body temperature 37.9 °C. The skin is pale pink, with elements of small, finely spotted rashes on the hyperemic background of the skin of the face, torso, limbs, especially pronounced in natural folds; visible mucous membranes are hyperemic. The tongue is moist, surrounded by a thick white fur with signs of epithelial desquamation on the surface. The submandibular lymph nodes are enlarged, elastic in consistency, not painful. By percussion the boundaries of the heart are age-dependent, auscultatory activity of the heart is rhythmic, the tones are sound, light systolic noise is present, heart rate is 96/min. The percussion of the lungs produces a clear pulmonary sound, there is vesicular respiration, and the breath rate is 22/min. The abdomen is symmetrical, not distended and soft on palpation. The liver protrudes 1 cm below the intercostal margin, its surface is smooth, elastic and it is not painful. Spleen is not enlarged.
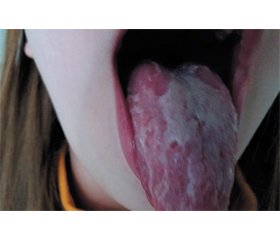
A dynamic observation was conducted, the spread and intensity of rashes increased. The girl’s face is slightly swollen, the cheeks are bright red, pallor of the nasolabial triangle was observed. At the objective examination of the oropharynx, a diffused hyperemia of the soft palate, tongue, and tonsils, the desquamation of the epithelium of the tongue are observed (Fig. 1).
The common clinical methods of examination were defined taking into consideration the pronounced exanthem syndrome on the background of the hyperthermia, scratches on the skin of the hands.
Blood tests showed anemia (Hb 10.5 g/dL), leukocytosis (white blood count 10.30/μl), neutrophilia (34.6 %), eosinophilia (12.0 %), increased levels of antistreptolysine O (994.0 IU/ml), the reactive protein was positive, and rheumatoid factor was negative.
/75_2.jpg)
Taking into consideration the characteristic clinical features, scarlet fever on the background of toxicodermia was diagnosed. Treatment was prescribed with intravenous systemic glucocorticoids at a dose of 3 mg/kg/day (prednisone), together with aminopenicillins (amoxicillin with clavulanic acid), antihistamines (levocetirizine), enterosorbents (silicon dioxide), as well as detoxification therapy (NaCl 0.9%, glucose 5.0%, rheosorbilact), vitamins (ascorbic acid, rutin), oral antiseptics (hexetidine, choline salicylate), skin treatment with topical glucocorticoids (clobetasol), hygienic care of the skin and mucous membranes, compliance with aseptic conditions when patient’s stay at home (frequent ventilation, quartz, daily change of clothes and patient’s bed linen). Nonsteroidal anti-inflammatory drugs (ibuprofen) were used to reduce body temperature. In the dynamics of treatment, the condition of the child has improved, there were no new rashes. Gradually, against the background of treatment, the elements of exanthem and enanthem regressed, sometimes with signs of peeling. The child was discharged from the hospital after 14 days, in a good clinical condition.
/76.jpg)
Discussion and literature review
Streptococcal infections are a group of diseases that can be caused by various serologic groups of streptococci, different in clinical manifestations and common in terms of epidemiology, pathogenesis, morphology and immunology [1–4]. Streptococcal agents cause the most common infections in childhood [2, 5]. The clinical manifestations of these diseases are quite diverse: from light inflammatory processes in mucous membranes, skin, subcutaneous fatty tissue to severe generalized forms that run by a type of septicemia, septicopyemia [2, 3, 6]. The group of streptococcal infections includes nosologies such as scarlet fever, impetigo contagiosum, pyelonephritis, glomerulonephritis, tonsillitis, pharyngitis, otitis, purulent meningitis, pneumonia, osteomyelitis [1–3, 6].
Scarlet fever is a severe infectious disease caused by the group A beta-hemolytic streptococcus (Streptococcus pyogenes), characterized by symptoms of general intoxication, acute tonsillitis, regional lymphadenitis, with a finely dotted rash on the skin, followed by residual peeling [2, 4, 7].
Streptococci are spheroidal Gram-positive microorganisms, belonging to the family of Streptococcaceae. Depending on the ability to lyse red blood cells, the streptococci are divided into β (complete hemolysis) [2, 4, 7], α (partial hemolysis) and γ (absence of hemolysis) ones [8, 9]. Nelson in 1981 created the most complete classification of streptococci. Based on the reaction, 21 streptococcal groups were singled out, which differ according to the carbohydrate component of their coating.
Each group is marked by a Latin letter — A, B, C, D, and others [2, 4, 5]. Among all these streptococcal groups, group A is on a special place, it includes Streptococcus pyogenes — β-hemolytic streptococcus. There are 80 serovars of β-hemolytic streptococcus, which are defined in agglutination reaction with the corresponding serums of immunized animals. Streptococci produce toxins, enzymes, hemolysins. Twenty ectocytic agents are identified by their hemolytic group A streptococcus. The most important for the clinic are erythrogenic toxins (A, B, and C) [1–7]. The streptococcal toxin has two fractions — heat-labile and heat-stable. The heat-labile fraction of the toxin is the most important pathogen product of the hemolytic streptococcus during scarlet fever. Apart from the toxic properties, streptococci synthesize enzymes: streptolysin (O and S), diphosphopyridine nucleotidases, streptokinase (A and V), deoxyribonuclease (A, B, C, and D), hyaluronidase, protease, lipase, esterase [2–8]. Group A streptococcal cell wall consists of M-, T- and R-proteins, which have antigenic properties. T-protein is pre–sent in all streptococcal strains, on this basis the streptococcal typing on the group is performed. M-protein provides pathogen fixation at the place of adhesion and causes its virulence [3, 7, 9]. Groups C and G streptococci have many virulent factors similar to S.pyogenes. Both groups produce streptolysin O and can stimulate an increase of antistreptolysin O titer. In addition, they produce degrading enzymes, including hyaluronidase [2, 3, 5].
Group A streptococcal infections are constantly found in the oropharynx in healthy people but only in 15.0–20.0 % of them they may be the cause of the disease. Incidence rates depend on the age, climatic conditions, season, crowding, and frequency of people’s contacts [4–8]. Streptococcus pyogenes sensitive to ampicillin, amoxicillin, azithromycin is found in the oropharyngeal swab of the patient. The child fell ill acutely, the above-mentioned clinical symptoms appeared two weeks after acute tonsillitis caused by a compromised allergic history, the previously noted toxic and allergic dermatitis.
In the clinical classification of scarlet fever, there are typical and atypical forms of three degrees of severity (mild, moderate-to-severe, severe); infectious-allergic or purulent complications are possible [1–5].
The diagnosis of scarlet fever is based on the typical clinical criteria of the incubation period, lasting from several hours to 7 days; onset of acute illness; intoxication syndrome: fever, malaise, headache, lethargy, vomiting. The rash appears on days 1–2 of disease. The spotty rash appears on the hyperemic skin. Places of overwhelming localization of a rash are the flexural surface of the limbs, anterior and lateral surface of the neck, lateral surface of the trunk, abdomen, inner surface of the hips, natural folds of skin. There is no rash on nasolabial triangle (Filatov symptom).
Rash is accumulated in skin folds (Pastia’s sign), linear hemorrhagic elements of rash appear. There is a positive “pinch” symptom with new rash elements after physical action on the skin. Hemorrhagic rash, finely-spotted, with cyanotic tinge, dry skin, white dermographism, follicular, lacunar, or necrotic tonsillitis, hyperemia of the soft palate (“burning”), the enanthem on the soft palate may develop. White coating appears on the tongue, and it is gradually cleared from the plaque from 2nd to 4–5th days of illness, then tongue becomes bright red with papillae. Regional submandibular or cervical lymphadenitis is being detected, as well as peeling of the skin from the end of the first week: on the face, neck, on the trunk, extremities, on the hands and feet; changes in phases of the autonomic nervous system: during the first 3–4 days — tachycardia, increased blood pressure, from days 4–5 — bradycardia, lower blood pressure. History data were also collected about contact with people diagnosed with scarlet fever or other streptococcal infection 2–7 days before the onset of symptoms, acute onset, sore throat — acute tonsillitis, presence of regional lymphadenitis, and an appearance of skin rash by the end of day 1 or on day 2. The blood test showed neutrophilic leukocytosis, eosinophilia, erythrocyte sedimentation rate acceleration, presence of IgM (early stage) and IgG (late stage) antibodies to beta-hemolytic streptococcus, the growth of antibody titer to streptococcal M-protein, streptolysin O. The bacteriological method helped detect the growth of beta-hemolytic streptococci in sowing material from a focus of infection. Additional instrumental methods of examination are used depending on the affected organs (radiography, electrocardiography, ultrasound diagnosis) [1–9].
The treatment of a patient with scarlet fever includes bed rest during the acute period (5–7 days), diet therapy (milk and vegetables). Targeted therapy includes broad-spectrum antibiotics: in mild form of disease — penicillins (amoxicillin with clavulanic acid, ampicillin with sulbactam), or macrolides (azithromycin, midecamycin, clarithromycin); in moderate-to-severe — penicillins (amoxicillin with clavulanic acid, ampicillin with sulbactam); in case of severe form of disease, first- or second-generation cephalosporins (сefazolin, сefuroxime), clindamycin, vancomycin are prescribed. Detoxification therapy is carried out by oral and parenteral glucose-saline solutions. Pathogenetic therapy includes antihistamines (cetirizine, levocetirizine), drugs that strengthen vascular wall (ascorbic acid, rutin), and nonsteroidal anti-inflammatory drugs (paracetamol, ibuprofen). Local sanation means gargarism using disinfection solutions of antiseptics [1–9].
Prevention of scarlet fever: children, who were in contact with a patient with scarlet fever, are observed for 7 days from the moment of contact. They can be allowed to visit children’s group activities after the additional 12-day isolation (total 22 days). Specific prevention of scarlet fever has not been developed yet [5, 7, 8].
Toxicodermia or toxic-allergic dermatitis is the acute inflammation of the skin and sometimes mucous membranes, which arises as a reaction when substances (allergens) that have simultaneously allergic and toxic effects enter the body by respiratory, endodermal, parenteral routes. Thus, the etiological factor does not directly contact the skin, like in case of contact dermatitis, and penetrates the skin through the blood. Exogenous and, less often, endogenous causes predominate in the etiology of toxicodermia [10, 13, 14].
Exogenous causes include medications, infections, food, industrial and household chemicals entering the body through the digestive and respiratory system or directly the bloodstream. Also, substances that may cause toxicodermia can penetrate through any method of their introduction: intravenous, intramuscular, subcutaneous, intradermal, and as a result of absorption through the skin when applied externally. The cause is also an autointoxication with unusual metabolic products and accumulation of autoallergens, which appear in the body due to the dysfunction of the gastrointestinal tract, liver, kidneys, thyroid gland; tumors; metabolic disease [10–14].
The basis for the pathogenesis of toxicodermia is an allergic reaction as a manifestation of the sensitizing effect of an exogenous substance (allergen). Pathogenesis of toxicodermia simultaneously combines toxic and allergic components, which causes the development of various lesions of the skin, mucous membranes, vascular system, internal organs [10, 12, 14].
When toxicodermia is classified by etiological factors, the following forms are present: drug, vaccination, food, autointoxication. Three degrees of severity are mild, moderate-to-severe, severe. The prevalence of rashes can be classified as localized, widespread, diffuse. Morphological elements of the skin rash in case of toxicodermia can be spotted, papular, maculopapular, urticarial, vesicular, bullous, nodular, pigmented, purple, bullous haemorrhagic [10–14].
Diagnosis of toxicodermia is based on typical clinical criteria (skin rashes, itching, peeling, burning, impaired skin sensitivity, tearing, gastrointestinal disturbances, fever, malaise, joint pain and headaches, numbness in the tongue, abdominal pain, palpitations, weakness, bronchospasm, nausea, vomiting, diarrhea), history data (recent drug intake, the presence of similar symptoms before taking medication, allergies in the past medical history, the effectiveness of previously conducted desensitizing therapy), findings of physical examination (characteristic skin lesions with polymorphic rash elements — stains, erythema, papules, blisters, nodules, vesicles, erosion, and hemorrhage, accompanied by elevated body temperature, headache, general poor self-esteem, nausea, eating disorders) [10, 12, 13], which were observed in our patient.
The blood analysis showed leukocytosis, eosinophilia, accelerated erythrocyte sedimentation rate, hypercoagulation, increased levels of IgE, and circulating immune complexes. To identify etiological factors, specific in vivo and in vitro allergy testing is used [11, 12]. The complexity of the differential diagnosis is due to the fact that symptoms of toxic and allergic dermatitis are typical for other diseases [10, 13]. The most difficult complications of toxicodermia are Lyell’s syndrome (toxic epidermal necrolysis), Stevens-Johnson syndrome, toxico-septic shock [12].
Treatment of toxicodermia. First of all, it is termination of the intake of all substances, which presumably could cause the disease, as an etiological factor [10, 11, 14]. Diet in severe allergy. Pathogenetic therapy: systemic glucocorticoids (prednisone, methylprednisolone, dexamethasone), antihistamines (loratadine, desloratadine, cetirizine, levocetirizine, chloropyramine, dimetindene), mast cell stabilizers (ketotifen, zaditen), enterosorbents (silicon dioxide, activated carbon), detoxification therapy (glucose-salt solutions, sodium thiosulfate). Local therapies of the affected skin include the use of topical glucocorticoids (clobetasol, betamethasone, methylprednisolone, mometasone, triamcinolone, prednisone, hydrocortisone, fluocinolone, desonide), adsorbing agents (zinc paste), combined preparations of toрical glucocorticoid with antibiotics, antifungals (betamethasone, gentamicin/natamycin, clotrimazole) [10–14].
Conclusions
Scarlet fever combined with toxicodermia is a rare clinical condition, the etiology, and pathogenesis of which are not thoroughly investigated. The pyrogenic exotoxins types A, В, C synthesized by beta-hemolytic streptococci are predisposing factors. Epidemiological and allergic history plays a crucial role in the diagnostic investigation and differential diagnosis in such clinical cases. In our opinion, the use of antibiotics, glucocorticoids, and topical treatment are optimal to stabilize the child’s condition, it supports a rapid regression of exanthem with enanthem and prevents the development of complications. Pediatricians should be attentive to streptococcal infection, which may recur and may cause combined infectious-allergic complications.
Author contributions: S. Nykytyuk — diagnostic procedures, clinical diagnosis, and treatment decisions, writing the manuscript; O. Mochulska — diagnostic procedures; S. Levenets — writing the manuscript; T. Vorontsova — collection of the articles/published data and their inclusion and interpretation in this review.
All authors contributed to the critical revision of the manuscript for valuable intellectual content. All authors have read and agreed with the published version of the manuscript.
Conflicts of interests. Authors declare the absence of any conflicts of interests and their financial interest that might be construed to influence the results or interpretation of their manuscript. The authors declare that all the procedures and experiments of this study were done in line with the ethical standards of the Declaration of Helsinki 1975, as revised in 2008(5), as well as the national law. Informed consent was obtained from the patient included in the study. No funding was received for this study.
/75.jpg)
/75_2.jpg)
/76.jpg)