Журнал «Практическая онкология» Том 4, №1, 2021
Вернуться к номеру
Оптимальна інтеграція інгібіторів CDK4/6 у лікування гормон-рецепторпозитивного метастатичного раку молочної залози
Авторы: Риспаєва Д.Е.
Лікарня ізраїльської онкології «LISOD», м. Київ, Україна
Рубрики: Онкология
Разделы: Справочник специалиста
Версия для печати
У статті проведено аналіз ефективності нового класу препаратів — інгібіторів циклінзалежних кіназ CDK4/6 з розкриттям їх механізму дії. Описано загальні результати основних досліджень щодо палбоциклібу, рибоциклібу та абемациклібу* в першій і наступних лініях терапії HR-позитивного/HER2-негативного метастатичного раку молочної залози (РМЗ). Наведено профіль побічних ефектів затверджених інгібіторів CDK4/6. Показано, що комбінація інгібіторів CDK4/6 з ендокринною терапією дає перевагу у виживаності, можливості подолання гормонорезистентості та великі перспективи в поліпшенні клінічних результатів лікування гормон-рецепторпозитивного метастатичного РМЗ.
The paper analyzes the effectiveness of a new class of medications — CDK4/6 cyclin-dependent kinases inhibitors and discover the mechanism of its action. The article describes the general results of studies on palbociclib, ribociclib and abemaciclib* for the first- and subsequent-line of the therapy of HR-positive/HER2-negative metastatic breast cancer. The profile of adverse events of approved CDK4/6 inhibitors are considered. The combination of CDK4/6 inhibitors with endocrine therapy was shown to have benefits in survival, potentiality to reduce hormone resistance and great perspectives to improve clinical results of hormone receptor-positive metastatic breast cancer.
метастатичний рак молочної залози; інгібітори CDK4/6; палбоцикліб; рибоцикліб; абемацикліб; ендокринна терапія; гормонорезистентність
metastatic breast cancer; CDK4/6 inhibitors; palbociclib; ribociclib; abemaciclib; endocrine therapy; hormone resistance
Вступ
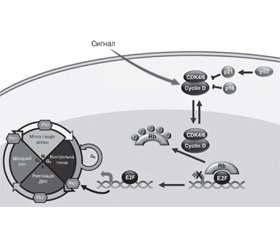
Механізм дії інгібіторів CDK4/6
Використання інгібіторів CDK4/6 у першій лінії терапії HR+/HER2– метастатичного РМЗ
Використання інгібіторів CDK4/6 у наступних лініях терапії HR+/HER2– метастатичного РМЗ
Що після інгібіторів CDK4/6? Шляхи подолання гормонорезистентності
Профіль безпеки інгібіторів CDK4/6
Висновки
- Siegel R.L., Miller K.D., Jemal A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017. 67(1). 7-30.
- Федоренко З.П. та ін. Бюлетень Національного канцер-реєстру України «Рак в Україні, 2017–2018». Київ, 2019.
- Nadji M. et al. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. Am. J. Clin. Pathol. 2005. 123(1). 21-7.
- Li C.I., Daling J.R., Malone K.E. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J. Clin. Oncol. 2003. 21(1). 28-34.
- Hess K.R. et al. Estrogen receptors and distinct patterns of breast cancer relapse. Breast Cancer Res. Treat. 2003. 78(1). 105-18.
- Dickson M.A. Molecular pathways: CDK4 inhibitors for cancer therapy. Clin. Cancer Res. 2014. 20(13). 3379-83.
- DeMichele A., Chodosh L.A. “Braking” the Cycle of Resistance in Endocrine Therapy for Breast Cancer. Clin. Cancer Res. 2015. 21(22). 4999-5001.
- Finn R.S. et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015. 16(1). 25-35.
- Hortobagyi G.N. et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016. 375(18). 1738-1748.
- Goetz M.P. et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017. 35(32). 3638-3646.
- Baker S.J., Reddy E.P. CDK4: A Key Player in the Cell Cycle, Development, and Cancer. Genes Cancer. 2012. 3(11–12). 658-69.
- Infante J.R. et al. A Phase I Study of the Cyclin-Dependent Kinase 4/6 Inhibitor Ribociclib (LEE011) in Patients with Advanced Solid Tumors and Lymphomas. Clin. Cancer Res. 2016. 22(23). 5696-5705.
- Hamilton E., Infante J.R. Targeting CDK4/6 in patients with cancer. Cancer Treat. Rev. 2016. 45. 129-38.
- Murphy C.G., Dickler M.N. The Role of CDK4/6 Inhibition in Breast Cancer. Oncologist. 2015. 20(5). 483-90.
- Shapiro G.I. Cyclin-dependent kinase pathways as targets for cancer treatment. J. Clin. Oncol. 2006. 24(11). 1770-83.
- Kwapisz D. Cyclin-dependent kinase 4/6 inhibitors in breast cancer: palbociclib, ribociclib, and abemaciclib. Breast Cancer Res. Treat. 2017. 166(1). 41-54.
- Ingham M., Schwartz G.K. Cell-Cycle Therapeutics Come of Age. J. Clin. Oncol. 2017. 35(25). 2949-2959.
- DeMichele A. et al. CDK4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin. Cancer Res. 2015. 21(5). 995-1001.
- Finn R.S. et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009. 11(5). R77.
- Finn R.S. et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016. 375(20). 1925-1936.
- Rugo H.S. et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res. Treat. 2019. 174(3). 719-729.
- Hortobagyi G.N. et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018. 29(7). 1541-1547.
- Johnston S. et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019. 5. 5.
- Rossi V. et al. Should All Patients With HR-Positive HER2-Negative Metastatic Breast Cancer Receive CDK4/6 Inhibitor As First-Line Based Therapy? A Network Meta-Analysis of Data from the PALOMA 2, MONALEESA 2, MONALEESA 7, MONARCH 3, FALCON, SWOG and FACT Trials. Cancers (Basel). 2019. 11(11).
- Cristofanilli M. et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016. 17(4). 425-439.
- Sledge G.W. et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017. 35(25). 2875-2884.
- Slamon D.J. et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: –MONALEESA-3. J. Clin. Oncol. 2018. 36(24). 2465-2472.
- Turner N.C. et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018. 379(20). 1926-1936.
- Sledg G.W. et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy — MO–NARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2019.
- Slamon D.J. et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2020. 382(6). 514-524.
- Di Leo A. et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J. Clin. Oncol. 2010. 28(30). 4594-600.
- Chia S. et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J. Clin. Oncol. 2008. 26(10). 1664-70.
- Cardoso F. et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann. Oncol. 2018. 29(8). 1634-1657.
- Baselga J. et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012. 366(6). 520-9.
- André F. et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019. 380(20). 1929-1940.
- Hamilton E.P. et al. A First-in-Human Study of the New Oral Selective Estrogen Receptor Degrader AZD9496 for ER+/HER2(-) Advanced Breast Cancer. Clinical Cancer Research. 2018. 24(15). 3510-3518.
- Curigliano G. et al. Phase 1/1b study of novel oral selective estrogen receptor degrader (SERD) LSZ102 in combination with alpelisib (BYL719) in estrogen receptor-positive (ER plus), human epidermal growth factor receptor-2-negative (HER2-) advanced breast cancer (ABC) with progression on endocrine therapy (ET). Cancer Research. 2019. 79(4). 3.
- Dees E.C. et al. Dose-escalation study of G1T48, an oral selective estrogen receptor degrader (SERD), in postmenopausal women with ER+/HER2-locally advanced or metastatic breast cancer (ABC). Annals of Oncology. 2019. 30. 121.
- Giuliano M. et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019. 20(10). 1360-1369.
- Verma S. et al. Palbociclib in Combination With Fulvestrant in Women With Hormone Receptor-Positive/HER2-Negative Advanced Metastatic Breast Cancer: Detailed Safety Analysis From a Multicenter, Randomized, Placebo-Controlled, Phase III Study (PALOMA-3). Oncologist. 2016. 21(10). 1165-1175.
- Spring L.M. et al. Clinical Management of Potential Toxicities and Drug Interactions Related to Cyclin-Dependent Kinase 4/6 Inhibitors in Breast Cancer: Practical Considerations and Recommendations. Oncologist. 2017. 22(9). 1039-1048.
- Hu W. et al. Mechanistic Investigation of Bone Marrow Suppression Associated with Palbociclib and its Differentiation from Cytotoxic Chemotherapies. Clin. Cancer Res. 2016. 22(8). 2000-8.
- Sammons S.L., Topping D.L., Blackwell K.L. HR+, HER2- Advanced Breast Cancer and CDK4/6 Inhibitors: Mode of Action, Clinical Activity, and Safety Profiles. Curr. Cancer Drug. Targets. 2017. 17(7). 637-649.
- Rugo H.S. et al. Endocrine Therapy for Hormone Receptor-Positive Metastatic Breast Cancer: American Society of Clinical Oncology Guideline. J. Clin. Oncol. 2016. 34(25). 3069-103.
- ClinicalTrials.gov., Study comparing two different schedules of Palbociclib plus second line endocrine therapy in women with estrogen receptor positive, HER2 negative advanced/metastatic breast cancer. 2016, Available at https://clinicaltrials.gov/ ct2/show/NCT02630693. Accessed April 25, 2016.
- Im S.A. et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019. 381(4). 307-316.
- Administration, U.F.a.D., FDA warns about rare but severe lung inflammation with Ibrance, Kisqali, and Verzenio for breast cancer. 2019.
- Barroso-Sousa R., Shapiro G.I., Tolaney S.M. Clinical Development of the CDK4/6 Inhibitors Ribociclib and Abemaciclib in Breast Cancer. Breast Care (Basel). 2016. 11(3). 167-73.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer version 2, 2020.
- Gao J.J. et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2020. 21(2). 250-260.
- Turner N.C. et al. Clinical considerations of the role of palbociclib in the management of advanced breast cancer patients with and without visceral metastases. Ann. Oncol. 2018. 29(3). 669-680.

/14.jpg)
/13.jpg)
/15.jpg)
/16.jpg)