Introduction
Fibronectin glomerulopathy (FNG) or glomerulopathy with fibronectin deposits (GFND) is a rare hereditary condition with an autosomal dominant inheritance. Burgin et al described this disease for the first time in 1980 year [1]. The main features of this glomerulopathy is immunoreactivity of glomerulus with a monoclonal immunoglobulin on serum fibronectin. Fibronectin glomerulopathy is diagnosed and confirmed with kidney biopsy. The deposits of fibronectin at first are deposited in the mesangium and then in subendothelial area. They are red with trichrome stain and stain intensely with the period acid Schiff (PAS) stain. The deposits are negative with the congo red T and S stains. The deposits are made of granular to occasional fibrillary material, with and thioflavins fibrils measuring 14 to 16 nm in diameter. Mutations of fibronectin gene (FN1) are located in 1q32 chromosome and timely diagnosis prevents this disease in the next probands. Progression to end-stage renal disease may be done slowly in some patients. Recurrence of disease after kidney transplantation has been documented [2]. The aim of this research is to assess effect of FN1 gene mutation as risk factor on progressive kidney failure. Confirmation by genetic analysis helps to the better recognition of the entity in the next probands and prevention of disease. Today’s science moves toward genomics detection of diseases.
Materials and methods
Among screened 5374 full-text articles obtained in this research paper, 5250 articles were excluded due to unrelated subject, review articles and other studies. Then 124 full-text articles were eligible and 100 articles were excluded due to non case report. All case reports were obtained via electronic search in PubMed central (PMC), PubMed and Google Scholar database. These 24 articles included 57 case reports that were examined 57 patients with fibronectin deposits in kidney biopsy and renal dysfunction for systematic review and meta-analysis synthesis. Patients with kidney disorders and pathologic characteristics of fibronectin deposition in kidney biopsy were considered for this research. Risk and odds of end-stage kidney disease progression (need to persistent hemodialysis) in patients with positive FN1 gene (as risk or contributing factor) and risk of graft loss after kidney transplant according to Banff 2013 classification were primary outcomes in this study. Decreased estimated glomerular filtration rate (eGFR), elevated urinary albumin creatinine ratio (UACR) for detecting proteinuria and mortality rate were secondary outcomes in this study. The paper has written based on advanced searching via PubMed central (PMC), PubMed and Google Scholar databases to identify published articles since inception to April 2022. The mentioned search used the following search terms of fibronectin nephropathy, GFND and advanced search with fibronectin and kidney. The author reviewed references of all included articles and performed handsearching of related journals to identify the additional relevant studies. The search strategy was used to obtain titles and abstracts of studies that might be relevant to the review. The 5383 titles and abstracts were screened via electronic search in PMC, PubMed and Google Scholar by author, respectively. Total records of 5383 articles were screened and after deduplication 5374 articles identified. Of them, 5250 articles were excluded due to non-related subject, review articles, others and 124 full-text articles were considered for eligibility. However studies and reviews that might include relevant data or information studies were retained initially. The 100 articles were excluded due to not case reports. Then 57 case reports in 24 published articles that were examined 57 patients with fibronectin deposits in kidney biopsy and renal dysfunction were included for qualitative and quantitative synthesis. Data extraction was carried out by author and studies that reported in non-English language journals were to be translated before assessment. Where more than one publication of one study existed, reports were viewed and studied then the publication with the most complete data was included. All patients with pathologic characteristics of fibronectin deposition in kidney biopsy with decreased eGFR were considered in this research. Clinical features such as age, sex, different symptoms and physical signs were extracted from this study. Furthermore, biochemical variables of serum creatinine (SCr), eGFR, urine protein, genetic testing at initial presentation, imaging, management and outcomes were collected. The most recent American Heart Association/American College of Cardiology (AHA/ACC) guidelines defines an office blood pressure (BP) < 120 mm Hg as normal, and office BPs in the range of 120 to < 130/80 mm Hg are defined to be high. An office BP ≥ 130/80 mmHg would account as the threshold for hypertension [3]. Three missense mutations have been seen in the fibronectin gene — W1925R, L1947R and Y973C that can lead to formation of abnormal cysteine (Cys) — Cys bonds and protein folding. Therefore, the fibronectin deposits are produced in the extracellular matrix (ECM) of the glomerulus [4]. Case reports were analyzed using criteria developed by the Joanna Briggs Institute Critical Appraisal tool for case reports that has different assessment tools for each study design in question. The evaluation tool has 8 items for case reports.
Statistical analysis
Data were entered in Microsoft Excel 2010 software. Categorical variables are recorded as frequency (N) and percentage (%). The continuous variables were determined as to whether they were normally distributed using the kolmogorove-smirnov or shapiro-wilk test. Continuous variables with normal distribution reported as mean ± standard deviation (SD). Nonparametric variables are expressed as median and interquartile range (Q1, Q3 and IQR). Comparisons between continuous variables with normally distributed (ND) data assessed by two-tailed one-sample t test analysis. Relative risk and Odds ratio for assessing effect measures of risk factor on outcomes of disease were used. Significance was assessed with p-value of < 0.05.
Results
Author identified total records of 5383 after searching through PubMed and Google scholar databases as electronic method and then screened 5374 articles after deduplication. During this research, 5250 articles were discarded due to unrelated subject and 124 articles became eligible. Thereafter, 100 articles excluded for non-case reports and to end 57 patients in twenty-four published articles were included and enrolled for participate in this present research (Fig. 1).
/8.jpg)
Twenty-four published articles (57 case reports or participants) were considered for inclusion in this research. Extracted data were planned with analytic (experimental) type of clinical studies with randomized clinical trials design in systematic review and meta-analysis article in the present research. Sample sizes ranged from 57 to 64 patients in this study that eight patients excluded from this study. Participants were referred to single center in eleven case reports and these situations were not mentioned in thirteen case reports. All patients included in this study had kidney diseases in relation with fibronectin deposition in kidney biopsy. Patients were excluded from the study if they were not diagnosed as fibronectin glome–rulopathy in kidney biosy specimens. Assessment of risk of bias and quality of included articles performed using Joanna Briggs Institute critical appraisal tools for case reports. In this research, eight of patients (8/57, 14 %) achieved 8 score, twenty-three of patients obtained seven score (23/57, 40.3 %), twenty-five of patients obtained six score (25/57, 43.8 %), one patient (1/57, 1.7 %) achieved four sore [Table S1*]. Among screened 5383 full-text articles obtained in this research paper, 5374 articles were excluded due to unrelated subject, review articles and other studies. Then 124 full-text articles were eligible and 100 articles were excluded due to not case report (n = 100). Finally 24 published articles were included in this study. These 24 articles included 57 case reports that were examined 57 patients with fibronectin deposition in kidney biopsy and renal dysfunction for qualitative and quantitative synthesis [Table S2]. Fifty-seven patients were enrolled in this research with median age of 30 with Q1 of 21, Q3 of 47.5 and IQR of 26.5 years old and age range of 4 to 88 years old. Thirty-six out of fifty-seven patients (36/57, 63.1 %) were male and twenty-one patients were female (21/57, 36.8 %). The mean average of age in male and female levels were assessed 35.80 ± 19.63 and 37.28 ± 18.63 years old, respectively. There wasn’t statistical significant level between two sex groups (p-value: 0.78). Twenty-three of fifty-seven patients (23/57, 40.3 %) were from Switzerland ethnicity, fourteen of fifty-seven patients (14/57, 24.5 %) from Japanese, six of fifty-seven patients from Italian ancestry (6/57, 10.5 %), four of fifty-seven patients from Chinese (4/57, 7 %), two of fifty-seven patients from USA, Netherland, Brazil (2/57, 3.5 %) and one patient (1/57, 1.7 %) from India, Burma and Caucasian race [Table S3]. Fourteen of fifty-seven patients were mentioned history of nephrotic syndrome and family history of proteinuria or renal dysfunction (14/57, 24.5 %), ten out of fifty-seven patients with history of proteinuria (10/57, 17.5 %), six of fifty-seven patients gave history of hematuria and hypertension (6/57, 10.5 %), five of fifty-seven patients had history of edema (5/57, 8.7 %), three of fifty-seven had history of kidney impairment (3/57, 5.2 %), two of fifty-seven mentioned history of chronic obstructive airway disease (2/57, 3.5 %) and one out of fifty-seven patients with unknown family history (1/57, 1.7 %) in this research [Table S4a-b]. In sign of patients, two of fifty-seven patients (2/57, 3.5 %) developed significant elevated weight and one of fifty-seven patients (1/57, 1.7 %) presented with tachycardia (pulse rate above 100 beat per minute) in this research. Twenty-three of fifty-seven patients (23/57, 40.3 %) had hypertension based on 2017 AHA/ACC guidelines, seven of fifty-seven patients (7/57, 12.2 %) revealed lower extremity edema, two of fifty-seven patients (2/57, 3.5 %) had peritoneal fluid and anemia, one of fifty-seven patients (1/57, 1.7 %) showed generalized, scrotal edema and bilateral pleural effusion [Table S5].
/9.jpg)
Blood urea nitrogen (Bun) level was measured in sixteen patients (16/57, 28.07 %) with fibronectin glomerulopathy that elevated Bun levels were seen in five of sixteen patients (5/16, 31.2 %) with the mean average of 115.34 ± 91.03 mg/dl. Serum creatinine level (SCr) was measured in forty-four patients (44/57, 77.1 %) and thirteen of forty-four patients (13/44, 29.5 %) showed elevated SCr with the median of 1.6 mg/dl (IQR of 0.98 mg/dl) in this research. Forty-eight of fifty-seven patients (48/57, 84.2 %) had proteinuria above 150 mg/day using 24-hr urine collection PER or spot UPCR or dipstick test from urinalysis or dipstick test from 24-hr urine collection sample and proteinuria was not measured in nine out of fifty-seven patients (9/57, 15.7 %) in this research. 24-hr proteinuria was measured in thirty-nine of fifty-seven patients (39/57, 68.4 %) and there was significant proteinuria in thirty-five out of thirty-nine patients (35/39, 89.7 %) with the median of 3.9 g/d (IQR of 6 g/day). Random spot UPCR was measured in eight out of fifty-seven patients (8/57, 14.03 %) and there was significant proteinuria in eight of fifty-seven patients (8/57, 14.03 %) in spot urine protein to creatinine ratio with the median of 3.49 g/g Cr (IQR of 4.75 g/g Cr). Proteinuria using urinalysis was measured in thirteen out of fifty-seven patients (13/57, 22.8 %) and twelve of thirteen patients (12/13, 92.3 %) revealed semiquantitative proteinuria with the median of 2.5+ (IQR of 1+). Dipstick test from a specimen of 24-hr urine collection was seen in four of fifty-seven patients (4/57, 7.01 %) with the mean average of 2.75 ± 1+ in this research. Nephrotic range proteinuria (> 3.5 g/day) from 24-hr urine collection sample was seen in seventeen of thirty-five patients (17/35, 48.5 %) with the median of 5.42 g/day (IQR of 3.37 g/24 hr). Nephrotic range proteinuria using UPCR specimen was seen in four out of eight patients (4/8, 50 %) with 8.86 ± 5.7 g/g in this research. Relative risk and Odds ratio of ESKD in nephrotic-range proteinuria versus non-nephrotic range proteinuria were assessed 1.33 and 1.39, respectively. Therefore it can be said that patients with nephrotic proteinuria have more risk to experience ESKD versus patients with non-nephrotic proteinuria (positive association). Moreover, they have more likely to experience ESKD with KRT than patients with non-nephrotic range proteinuria. Serum albumin levels were measured in sixteen out of fifty-seven patients (16/57, 28 %) and quantitative serum albumin levels were measured in fourteen out of fifty-seven patients (14/57, 24.5 %). There was hypoalbuminemia in seven of fourteen patients (7/14, 50 %) with the mean average 2.15 ± 0.57 g/dl in this research. Hematuria was measured in twenty-one of fifty-seven patients (21/57, 36.8 %) in urinalysis. Seventeen of twenty-one patients (17/21, 80.9 %) showed hematuria (red blood cells) and two of twenty-one patients (2/21, 9.5 %) revealed positive occult blood in urinalysis in initial presentation. Estimated GFR and Cr clearance were measured in six out of fifty-seven patients (6/57, 10.5 %) in this research. Decreased eGFR found in three of six patients (3/6, 50 %) with and hyperfiltrating eGFR was seen in one out of six patients (1/6, 16.6 %) in this research. Two out of six patients (2/6, 33.3 %) had normal eGFR with the mean average of 101.69 ml/min/1.73 m2. Creatinine clearance was measured in six out of fifty-seven patients (6/57, 10.5 %) and decreased CrCl detected in one of six patients (1/6, 16.6 %) in this research. Three of six patients had normal values of CrCl (3/6, 50 %) with the mean average of 102.33 ± 6.34 ml/min and two of six patients (2/6, 33.3 %) developed increased CrCl with the mean average of 126.5 ± 3.5 ml/min. Anti-nuclear antibody was measured in thirteen out of fifty-seven patients (13/57, 22.8 %) and three of thirteen patients (3/13, 23 %) had positive antinuclear antibody (ANA). Total cholesterol level was measured in ten out of fifty-seven patients (10/57, 17.5 %) and there was total hypercholesterolemia in seven of ten patients (7/10, 70 %) but quantitative total hypercholesterolemia detected in six of ten patients (6/10, 60 %) with mean average of 341.71 ± 53.2 mg/dl. Hemoglobin was measured in five out of fifty-seven patients (5/57, 8.7 %) and anemia found in four of five patients (4/5, 80 %) with mean average of 9.2 ± 0.75 g/dl in this research [Table S6a-g]. Kidney biopsy was performed in all patients (57/57, 100 %) in this research. Pathologic characteristics of patients with fibronectin glomerulopathy include Glomeruli (g), vessels (v), tubules (t), interstitium (i) and peritubular capillaries (ptc). In light microscopy (LM), thirty-eight of fifty-seven patients (38/57, 66.6 %) had enlarged glomeruli, fourteen of fifty-seven patients showed lobular appearance in their glomeruli (14/57, 24.5 %), eight of fifty-seven patients (8/57, 14 %) had sclerotic glomeruli. Tubular atrophy in five of fifty-seven patients (5/57, 8.7 %) and interstitial fibrosis in three of fifty-seven patients (3/57, 5.2 %) were seen. In immunofluorescence (IF), deposition of immunoglobulins, complements and light chains were seen in fifty-one of fifty-seven patients (51/57, 89.4 %) in this research. Immunoglobuin deposition was not seen in six of fifty-seven patients (6/57, 10.5 %). In electron microscopy (EM), extensive granular deposits in mesangial and subendothelial space were seen in twenty-two of fifty-seven patients (22/57, 38.5 %) and fibrillar deposits were seen in two out of fifty-seven patients (2/57, 3.5 %). Mesangial deposition was seen in two out of fifty-seven patients (2/57, 3.5 %), mesangial and subepithelial deposition was seen in one out of fifty-seven patients (1/57, 1.7 %), mesangial and paramesangial deposition found in one out of fifty-seven patients (1/57, 1.7 %) in EM. The size of fibrils was measured in fourteen of fifty-seven patients (14/57, 24.5 %) with mean average of 13.18 ± 3.63 nm in this research [Table S7]. A co-operative clinico-genetic study by clinicians in charge of surviving members of the mentioned families will contribute to the understanding of the pathogenesis of the nephropathy and will help to preventing affecteds of this inherited disease in future probands. Genetic testing was performed in sixteen of fifty-seven patients (16/57, 28 %) that fourteen of sixteen patients (14/16, 87.5 %) had positive genetic testing for FN1 mutation while it was negative in two of sixteen patients (2/16, 11.1 %). Eight of them (8/14, 57.1 %) were heterozygous and seven of them (7/14, 50 %) had missense mutation. Two of fourteen patients (2/14, 50 %) had deletion in FN1gene [Table S8]. There was cardiomegaly in chest x-ray of one patient in this research. Kidney ultrasonography was performed in eight out of fifty-seven patients (8/57, 14 %) and there was normal kidney sonography in eight of eight patients (8/8, 100 %) with fibronectin nephropathy in the present study. One patient revealed thymoma in chest computed tomography (CT) scan and another patient showed lipomatose liver and kidney cyst in abdominal CT scan [Table S9]. Seven of fifty-seven patients (7/57, 12.2 %) consumed angiotensin II receptor blockers and four of fifty-seven patients (4/57, 7 %) used angiotensin converting enzyme inhibitors (ACEis) in the current research. Treatment with term of renin angiotensin system inhibitors (RASis) used in one of fifty-seven patients (1/57, 1.7 %) in the present research. Oral prednisolon was used in six of fifty-seven patients (6/57, 10.5 %). Oral mizoribine consumed in two of fifty-seven patients (2/57, 3.5 %) in this research. Seven of fifty-seven patients (7/57, 12.2 %) underwent hemodialysis and eight of fifty-seven patients (8/57, 14 %) became kidney transplantation [Table S10a-b]. Serum creatinine level was measured in twenty-two of seventy-seven patients (22/57, 38.5 %) and elevated serum creatinine was seen in eleven of twenty-two patients (11/22, 50 %) in this research. Ten of twenty-two patients (10/23, 45.4 %) found elevated SCr with the mean average of during follow up period. Seven of fifty-seven patients (7/57, 12.2 %) persisted on kidney failure with kidney replacement therapy (hemodialysis). Relative risk and Odds ratio of ESKD with KRT in patients with positive FN1 gene (as risk or contributing factor) versus patients with negative FN1 gene was assessed 0.66 (95% confidence interval of 0.126‒3.52) and 0.50 (95% confidence interval of 0.022‒11.08), respectively. Therefore, there was negative association between patients with positive FN1 gene and ESKD with KRT. Moreover, patients with negative FN1 gene achieved less to ESKD with KRT than patients with positive FN1 gene. Comparison of patients of ESKD with KRT in positive FN1 gene versus ESKD patients with KRT in negative FN1 gene was assessed with p-value of 0.39 (Table 1). Recurrence of fibronectin glomerulopathy after kidney transplantation was seen in three of eight patients (3/8, 37.5 %) in current research. Recurrence proportion (fixed effect) of fibronectin glomerulopathy after kidney transplant was assessed 12.25 % with 95% confidence interval of 3.38 to 28.69. Random effect of FGN after kidney transplantation was assessed 26.66 % and 95 % confidence interval of 1.19 to 68.59 (Fig. 2).
/11.jpg)
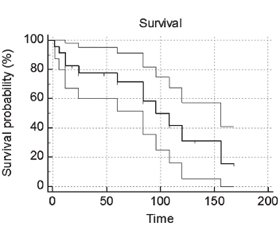
Risk of recurrence after kidney transplant with nephrotic-range proteinuria versus non-nephrotic proteinuria was assessed 0.83 (95% confidence interval of 0.1213 to 5.7244). Therefore, there was negative association between recurrence and nephrotic-range proteinuria in current research. Odds ratio of recurrence after kidney transplant with nephrotic proteinuria versus non-nephrotic range proteinuria was assessed 0.75 (95% confidence interval of 0.03757 to 14.9733) in current research. This findings means nephrotic-range proteinuria have 0.75 percent more odds to experience recurrence post-transplant (0.75 %). Effect of nephrotic-range proteinuria on recurrence after renal transplant versus non-nephrotic range proteinuria patients was assessed 100 % (3/3) versus 50 % (1/2) with proportion difference of 50 % and p-value of 0.22 (Table 2). Decreased eGFR using creatinine were decreased in four of fifty-seven patients (4/57, 7 %) with mean average of 64.25 ± 13.86 ml/min/1.73 m2 in this research. Proteinuria using 24-hr urine collection was measured in thirteen of fifty-seven patients (13/57, 22.8 %) during follow up and quantitative value was not mentioned in two of thirteen patients (2/13, 15.3 %). The mean average of significant proteinuria (> 150 mg/d) during follow up was assessed 2.94 ± 2.22 g/day in this research. Nephrotic-range proteinuria was seen in one of four out of thirteen patients (4/13, 30.7 %) with the mean average of 5.37 ± 1.63 in this research. Proteinuria in spot UPCR specimens were measured in four of fifty-seven patients (4/57, 7.01 %) with mean average of 5.00 ± 3.02 g/g Cr that this value decreased in one of four patients (1/4, 25 %) in this research. Two out of four patients (2/4, 50 %) found nephrotic-range proteinuria using spot UPCR with the mean average of 7.76 ± 1.76 g/g. Five of fifty-seven patients (5/57, 8.7 %) passed away during follow up [Table S11]. Probability of renal survival in nephrotic-range proteinuria was higher than patients with non-nephrotic proteinuria using Kaplan-Miere analysis. Probability of decreased kidney function in nephrotic-range proteinuria was assessed 45.4 % (5/11) versus patients with non-nephrotic proteinuria with assessment of 58.3 % (7/12). Comparison between decreased renal function in nephrotic-range proteinuria (group I) and non-nephrotic proteinuria (group II) was assessed nonsignificant with p-value of 0.54 with 95% CI of –24.5 to 45.82 (Fig. 3).
/12.jpg)
/12_2.jpg)
Discussion
Fibronectin glomerulopathy is an inherited disorder with extensive deposition of fibronectin substance in the glomeruli. It is may be as primary glomerulopathy or be secondary to a metabolic defect as recurrence of the disease after kidney transplantation. It has been described in Caucasians and Asians. In most patients progression to kidney failure is slow [5]. This research revealed 63.1 % of patients were male and 36.8 % belonged to female group. Median age of patients in this research was 30 years old that these findings were approximately in agreement with study by Zhang et al that 57.8 % of patients were male and 42.1 % were female [6]. Fibronectin glomerulopathy manifests with proteinuria after age of 20 years old. Eighty-four percent (84.2 %) of patients presented with significant proteinuria in our research and nephrotic range proteinuria accounted 48.5 % of it while this value in study by Zhang et al. was reported 73.6 %. Furthermore, RR and OR of nephrotic-range proteinuria as a risk factor in progressive renal failure to KRT was more than 1 in our study. This means that there is positive association between nephrotic-range proteinuria and risk of progressive kidney and patients with nephrotic-range proteinuria have more chance to achieving to ESKD in current research. Fourteen of sixteen patients (87.5 %) revealed mutations in FN1 gene in our research while mutations of FN1 gene were detected in two of nineteen patients (10.5 %) in Zhang et al. study. Another important point in current research is role of FN1 gene in achieving to ESKD with KRT. Our research showed negative association between FN1 gene as risk factor or contributing factor and risk of ESKD. Moreover, patients with positive FN1 gene showed fewer odds in achieving to ESKD with KRT in current research. Another important result in this research is recurrence of FNG after kidney transplantation. Risk of recurrence after kidney transplantation in patients with nephrotic-range proteinuria was assessed 0.83 in current research that few cases in literature review have been reported sofar. According to existing data and rarity of FNG, it must be talked that fibronectin glomerulopathy is a disease that may be missed without pathologic findings and genetic testing. Differential diagnosis of this disease are amyloidosis, fibrillary glomerulonephritis (FGN), immunotactoid glomerulonephritis (ITGN) and collagenofibrotic glomerulopathy (CG). Normal GBM comprise collagen type 4, 5 and 6 and type III collagen is usually seen in the interstitium and blood vessels throughout the body but it is not found in the glomeruli. Elevated blood levels of N-terminal propeptide of type III procollagen (PIIINP) accumulation of similar collagen fibers in other organs including liver, spleen, myocardium and thyroid gland is indicative of systemic nature of disease. So source of collagen III fibers may be mesangial cells or as a part of systemic disorder with secondary mesangial deposits [7]. CG is an inherited autosomal recessive disorder and it is diagnosed by marked elevated levels of type III procollagen peptides in serum and accumulation of type III collagen in mesangium and subendothelial areas in kidney biopsy. DNAJB9 is an autoantigen and immunohistochemical marker for diagnosis of fibrillary glomerulonephritis in EM of kidney biopsy specimens. This marker is a novel proteomic biomarker for FGN: Dnaj homolog subfamily B member 9 that is a member of the molecular chaperone gene family [8]. Differences between FGN and ITGN is based on appearance, diameter and pattern of microfibrils or microtubules in EM, type of immunoglobulin deposits (monoclonal or polyclonal IgG), serum total complement levels (hypocomplementemia in ITGN) and incidence of other systemic diseases (low in FGN vs. high in ITGN). Therefore correct diagnosis can be achieved by close understanding of these diseases, nature of inheritance and specific serum and tissue markers. Lack of access to full-text articles of eight papers are accounted for limitations in this research. They existed as abstract form and did not put in this research [Bayder, Chen, Sato, Bric, Niimi, Nadamuni, Zhang, Gemperle et al.].
Conclusions
Relative risk and odds ratio of end-stage kidney disease in nephrotic-range proteinuria versus non-nephrotic range proteinuria were assessed 1.33 and 1.39, respectively.
Acknowledgements. The author to wish thanks National University of Tehran Medical Sciences, College of Medicine and Imam Khomeini Hospital Complex. This paper has been written for medical students and higher degrees.
Availability of data and supplementary online material. Author requested that the datasets be located in figshare repository.
Received 04.02.2022
Revised 16.02.2022
Accepted 20.02.2022
Список литературы
1. Burgin M., Haffmann E., Reutter F.W. Familial glomerulopathy with giant fibrillary deposits. Virchows Arch. A Pathol. Anat. Histol. 1980. 3. 313-26. doi: 10.1007/BF00430861.
2. Herrera G.A., Turbat-Herrera E.A. Renal diseases with organized deposits-An algorithmic approach to classification and clinicopathologic diagnosis. Arch. Pathol. Lab. Med. 2010. 134. 512-31. DOI: 10.1043/1543-2165-134.4.512.
3. Ku E., Lee B.J., Wei J., Weir M.R. Hypertension in CKD: Core curriculum 2019. Am. J. Kidney Dis. 2019. 74. 12031. 10.1053/j.ajkd.2018.12.044.
4. Raparia K., Usman I., Kanwar Y.S. Renal morphologic lesions reminiscent of diabetic nephropathy. Arch. Pathol. Lab. Med. 2013. 137. 351-9. Doi: 10.5858/arpa.2012-0243-RA.
5. Thomas P.P. Fibrillary, immunotactoid and fibronectin glomerulopathies. In: Nur Elhuda̕s Text Book of glomerular diseases, 2012. 581-9.
6. Zhang T., Zhang W., Zuo K., Cheng Z. Clinicopathologic features and outcomes in fibronectin glomerulopathy: A case series of 19 patients. Front. Med. 2020. 7. 1-7. 10.3380/fmed.2020.00439.
7. Modi S.S., Balasubramanium S., Sunikumar K. Collagenofibrotic glomerulopathy: A case of glomerular deposition disease in the indian subcontinent and review of the literature. Saudi J. kidney Dis. Transpl. 2020. 31. 681-6. https://www.sjkdt.org/text.asp?2020/31/3/681/289454.
8. Nasr S.H., Vrana J.A., Dasari S., Bridoux F., Fidler M.E., Kaaki S., et al. DNAJB9 is a specific immunohistochemical marker for fibrillary glomerulonephritis. Kidney Int. Rep. 2018. 3. 56-64. http://dx.doi.org/10.1016/j.ekir.2017.07.017.
/12.jpg)

/8.jpg)
/9.jpg)
/11.jpg)
/12_2.jpg)