Oxidative stress is a process of damage by reactive oxygen species (ROS) to the tissues and organs at the cellular level, which occurs as a result of imbalance of peroxide homeostasis [1, 2].
This process can be the result of both a lack of antioxidant protection (AOP) caused by disruption of endogenous antioxidant production, and the formation of excessive amounts of free radicals (FR) [1].
Free radical is a molecule or a part of it that has an unpaired electron in an outer atomic or molecular orbit. FR can be neutral or charged — the so-called ion radicals [2]. ROS initiate free radical oxidation (FRO), one of the fundamental processes that ensure the normal functioning of any organism [1].
The Mitochondrial Free Radical Theory of Aging suggests that mitochondria are both the primary sources of ROS and the primary targets for ROS. Mitochondrial damage is a sign of several aging phenotypes, while the accumulation of mitochondrial DNA deletions induces primary mitochondrial damage by accelerating aging. Mitochondrial AOP has a positive effect on life expectancy and mitigates the changes associated with aging [3–8].
ROS are constantly formed in the oxidation process the main source of which is oxidoreductase and autooxidation of low molecular weight substances such as catecholamines [9].
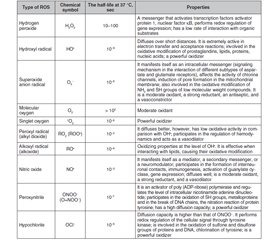
ROS include radicals such as superoxide anion (O2•), hydroxyl radical (HO•), nitric oxide (NO•), etc., and non-radical molecules with high reactivity: hydrogen peroxide (H2O2), singlet oxygen (1O2), ozone (O3), hypohaloids (HOCl, HOCI, HOBr, HOI, HOSCN), and others (Table 1). These are small molecules that have high reactivity due to the presence of an unpaired electron at the external electronic level [1, 2, 10, 11].
The primary oxidant is oxygen [11]. Molecular oxygen in the ground state has two unpaired electrons in the outer orbit with the same spin quantum numbers (biradical). Oxidation of any substance by an oxygen molecule is accompanied by filling its outer orbit with a pair of electrons that have parallel spins. This limits the reactivity of molecular oxygen, spin inhibition, so the oxidation processes are relatively slow [12, 13].
In the reactions of metabolism with O2, a primary radical is formed — the superoxide anion. The sources for its formation are enzymes such as nicotinamide adenine dinucleotide phosphate (reduced form) (NADPH) oxidase, lipoxygenase, and cyclooxygenase (enzymes of the arachidonic acid cascade), components of the respiratory chain of mitochondria, and electron-transporting smooth endoplasmic reticulum, xanthine oxidase, when involved in purine catabolism, etc. (Table 2) [11].
Also, the source of superoxide anion can be non-enzymatic processes such as autooxidation of cyclic unsaturated compounds (for example, catecholamines, hydroquinolones, flavoproteins), glucose and glycation reactions (proteins, lipids, and nucleic acids) [11].
The superoxide anion is rapidly converted to hydrogen peroxide, with the participation of superoxide dismutase (SOD) or spontaneously, can combine with other reactive molecules. Superoxide anion with transition metal cations (Fenton reaction) or with hydrogen peroxide (Haber-Weiss reaction) forms highly reactive hydroxyl radicals NO• [11].
The hydroxyl radical is very toxic. It denatures protein molecules, can cause the formation of inter- and intramolecular crosslinks due to oxidation of the SH group, which can change the tertiary structure of the protein [11].
Hydroxyl radical in interaction with lipid components of membranes initiates lipid peroxidation (LPO) with the formation of lipid radicals (L•), alkoxyls (LO•), hydroperoxides (LOON), peroxyls (LOO•), which causes cell dysfunction and death [11].
The most sensitive substrates for FRO are polyunsaturated fatty acids the oxidation products of which are hydroperoxides (ROOH) and peroxides (ROO•). LРO occurs in four stages: initiation, continuation, branching and breakage of the chain [11].
Initiation: the hydroxyl radical penetrates freely into the lipid layer due to the neutral charge and reacts with polyunsaturated acids forming lipid radicals, which in turn interacts with molecular oxygen to form a new radical — lipoperoxide (LOO•) [11].
Continuation: lipoperoxide interacts with neighboring phospholipid molecules, resulting in the formation of lipid hydroperoxide (LOOH) and a new radical L'•. The alternation of the last two reactions is a chain reaction of LPO [11].
Chain branching: in the presence of F2+, due to its interaction with lipid hydroperoxides (Fenton reaction), chain branching occurs with the formation of alkoxyl radicals (LO•), which initiate new chains of lipid oxidation [11].
Chain breakage: when radicals interact with antioxidants, metal ions of variable valency, or when interacting with each other [11].
Substances formed in the process of LPО can be divided into three classes: 1) lipid peroxides (13-hydroxyoctadecadienoic acid, etc.); 2) reactive lipids with electrophilic properties (4-hydroxynonenal, etc.); 3) receptor agonists (lysophosphatidylcholine, nitrolinoleic acid, etc.) [11].
LРO products (eg, 4-hydroxynonenal) have direct toxicity, can form derivatives with non-lipid compounds — DNA and proteins, disrupting membrane-associated signaling pathways, contributing to DNA damage [11].
LPO is evaluated by intermediate and final products: at the initial stages — hydroperoxides and peroxides, at subsequent stages — malonic dialdehyde (MDA), and as final products of LPO — Schiff bases, compounds formed by the interaction of MDA with free amino groups of biological molecules [11].
Some non-enzymatic antioxidants (Table 3) interact with FR and FRO catalysts (for example, transition metal ions) due to the mobile hydrogen atom. The mobility of a hydrogen atom is due to the unstable bond between the C-H and S-H atoms. As a result, there are inactive radicals of the antioxidant itself, which are unable to continue the chain reaction, inactive and excreted from the body [11].
Sometimes antioxidants do not break but slow down the chain reaction. Such AOР is directed against all types of radicals formed in a cell. Other non-enzymatic antioxidants (such as ascorbic and lipoic acids), when interacting with the oxidant, go from reduced to oxidized; the activity of the corresponding enzymes is required for the regeneration of the initial form of these compounds [11].
Chelate compounds such as uric acid, ferritin, transferrin, hemosiderin, and others play an important role in the non-enzymatic antioxidant system. They are able to neutralize FRO catalysts by binding transition metal ions [11].
Fat-soluble non-enzymatic antioxidants such as ubiquinone, estrogens, vitamin A, vitamin E, and carotenoids have impact on the biological membrane. On the other hand, water-soluble agents: urea, vitamin C, glutathione, lactoferrin, ferritin, lipoic acid, bioflavonoids, transferrin [11] act in the intercellular fluid, cell cytoplasm, lymph and blood plasma.
Glutathione tripeptide (L-γ-glutamyl-L-cysteinyl-L-glycine) plays an important role in the antioxidant system, which exists in the cell in 2 forms: reduced and oxidized (glutathione disulfide) [11].
Glutathione restores and isomerizes the –S–S– bonds by influencing the activity of enzymes by oxidizing SH groups of enzymes such as Ca2+ — adenosine triphosphatase, adenylate cyclase, pyruvate kinase, glucose-6-phosphatase [11].
There are four levels of protection of the cell by enzymatic antioxidants from the action of ROS. The first line of defense is SOD, converting the superoxide anion into hydrogen peroxide and molecular oxygen. The second line of defense is carried out by enzymes: catalase, glutathione peroxidase, and peroxiredoxin. Hydrogen peroxide with the participation of catalase decomposes into molecular oxygen and water, and with the participation of glutathione peroxidase and glutathione — into water and glutathione disulfide. The third line carries out the reduction of organic hydroperoxides with the formation of water and glutathione disulfide. This reaction is catalysed by two enzymes — glutathione peroxidase and glutathione transferase, which is unable to affect hydrogen peroxide, and restores only ROOH [11].
If the first three lines of defense are needed to reduce or prevent the progression of LPO and oxidative modification of proteins and nucleic acids, the fourth line is involved in the neutralization of metabolites of oxidative modification. Glutathione transferase combines a number of oxidized compounds with reduced glutathione such as 4-hydroxyalkenals and harmful epoxides, and aldehyde dehydrogenase oxidizes MDA [11].
For the last three lines, the auxiliary enzyme is glutathione reductase, which regenerates glutathione from glutathione disulfide by NADPH-dependent reduction. Thioredoxin and glutaredoxin proteins are important for the body’s antioxidant system. They are involved in the reduction of intramolecular and intermolecular disulfides [11].
Thioredoxin and glutaredoxin have two cysteine residues in the active site, and can exist in two redox forms: oxidized (–S–S–) and reduced (2SH–). 2SH forms react with disulfides and reduce them to free thiol groups. At the same time, they turn into –S–S– forms. With the help of thioredoxin reductase and coenzymes (restored flavin adenine dinucleotide) FADН2 and NADPH (for thioredoxin) or reduced glutathione, glutathione reductase and NADPH (for glutaredoxin), –S–S– forms are restored to the initial state of 2SH. NADPH for these reactions is supplied by pentose phosphate [11].
The effectiveness of the antioxidant system can be assessed by the activity of SOD, catalase, glutathione peroxidase, glutathione reductase, the content of reduced and oxidized glutathione, ascorbic acid, retinol, tocopherol, vitamin F, ceruloplasmin, etc. [11].
Under physiological conditions, ROS produced by phagocytic cells have bactericidal and immunomodulatory effects, participate in the detoxification of xenobiotics and protect the body from pathogens, tumor cells [1, 9, 14].
Under physiological conditions, ROS are formed mainly in the following systems:
1) in small quantities (up to 100 pmol) in the respiratory chain of mitochondria due to the transfer of 5–10 % of electrons from physiological acceptors to molecular oxygen. In this case, the 2amo acceleration O2•– is mainly generated, its rate directly depends on the degree of conjugation of the respiratory chain. Рhysical activity (muscle contraction), energy-dependent processes in the kidneys, transmembrane processes, etc. can activate the enzymatic complexes of the respiratory chain of mitochondria, which generate O2•– (NADP-dependent dehydrogenase, NAD-dependent ubiquinone reductase); O2•– is believed to be a precursor of all other forms of ROS in vivo [9, 15];
2) in the process of activation of NADPH oxidase. Blood phagocytes, chondrocytes, endotheliocytes, and astrocytes are characterized by the expression of this enzyme; activation of NADPH oxidase under the action of cytokines (interleukin 1 beta, interferon gamma, tumor necrosis factor beta, some growth factors) is accompanied by the formation of O2•– and H2O2; NADPH oxidase catalyzes 2amo, accelerates the reduction of O2, taking the reducing equivalent from NADPH [9, 51];
3) in the synthesis of prostaglandins (Pg) both in the lipoxygеnase pathway — in the process of conversion of arachidonic acid hydroperoxide into hydroxy acid, and in the cyclooxygenase pathway — in the process of PgG2 conversion to PgH2 (peroxidase function of PgH synthase); some cytokines (tumor necrosis factor beta), growth factors, peptide hormones (angiotensin) control this process [9, 16];
4) in the system of myeloperoxidase — H2O2-halogens (Cl–, Br–, I–), which is triggered due to the phagocytosis activation and promotes the formation of O2•–, OСl– and НО [9];
5) with monoamine oxidase-mediated oxidation of adrenaline and dopamine (during spontaneous oxidation, O2•– is formed, and during catalysed — H2O2) [9];
6) when activating glutamate receptors; activation of N-methyl-D-aspartate subtype of glutamate receptors on the postsynaptic membrane opens channels permeable to K+ and Ca2+. Intracellular production of ROS (O2•– and NO) is a consequence of activation of these receptors, and the result of activation of Ca2+-dependent NO synthase is NO [9];
7) when activating α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptors based on mitochondrial and Ca2+-dependent mechanisms [9];
8) during the synthesis of NO [9].
In the regulation of metabolic processes associated with protein phosphorylation, induction of Ca2+ signal, modulation of transcription factors, hydrolysis of phospholipids, ROS play an important role as secondary messengers, whilst hydrogen peroxide under optimal conditions acts as a signal molecule [1, 9, 17].
In the presence of transition metals such as copper and iron, hydrogen peroxide present in the cell promotes the formation of hydroxyl radicals, which are much stronger oxidants than hydrogen peroxide itself. The feature of hydrogen peroxide, in contrast to superoxide, to diffuse freely through the cell membrane increases the damaging effects of FRO [9, 10].
The concentration of superoxide, hydrogen peroxide in the cell is maintained at a very low level but at the same time is not equal to zero [13, 17]. Low concentrations of hydrogen peroxide cause a mitogenic effect and imitate the action of growth factors [9].
Hydrogen peroxide is a small molecule (electroneutral) that is rapidly produced in response to extracellular stimulation and is rapidly degraded by numerous mechanisms, including catalase [9, 17].
Since hydrogen peroxide is a secondary messenger in a cell (for signal transduction and enhancement), catalase is not only an enzyme of the AOP but also a factor influencing signal transduction in the cell [9].
The stability of hydrogen peroxide depends on the redox balance and pH in the cell. Hydrogen peroxide, in comparison with the hydroxyl radical and the superoxide anion radical, is a fairly mild oxidant, which primarily oxidizes cysteine residues in certain proteins. The spatial position of cysteine next to the polar anion of the acid makes it available for oxidation and provides selectivity of signaling only to certain proteins [9, 10].
In any stressful reactions accompanied by oxidative stress, ROS are involved in the transmission of information from primary mediators: hormones, cytokines, neurotransmitters across the cell membrane to trigger reactions of body adaptation to extreme conditions [1, 18].
Increased production of FR contributes to the development of endothelial dysfunction with dominating vasoconstrictive effects [19].
An indicator of FRO activation is the determination of the content of oxidatively modified proteins [9].
FRO is necessary for further renewal of cells and tissues in adapting to changing environmental conditions. Its second function is protection against infections. The third function — it is involved in the formation of biologically active compounds, including Pg [9].
FRO can lead to endogenous intoxication and an increase in the content of middle molecules (MM) with a molecular weight of 300–5000 Da. The concentration of MM increases with endotoxemia of various origins (toxaemia, burns, myocardial infarction, circulatory shock, uraemia, cancer) and the level of MM correlates with the severity of the disease [9].
MM play the role of endotoxins, being the products of degradation of proteins and their complexes and, by changing the physicochemical properties of membranes, make them more vulnerable to damaging actions, including LPO processes. Oxidative modification is one of the mechanisms of destruction of cellular structures and enzymes, followed by renewal of molecular components [9, 20].
Conjugated dienes, MDA and shifts in serum are products of LPO and reflect the activity of free radical oxidation processes [19, 21–23].
The effectiveness of AOP can be assessed by the activity of SOD, which binds ROS with the formation of hydrogen peroxide, as well as glutathione peroxidase and catalase [19, 24, 25].
LPO is a process in which lipids, primarily polyunsaturated fatty acids, are attacked by FR. Among the secondary products of LPO, the most studied one is MDA [19, 26].
MDA is one of the most reliable markers of oxidative stress in the clinic [19]. Due to the formation of Schiff bases, end products of LPO, MDA destabilizes membranes, promoting their destruction, stimulates platelet aggregation, increases platelet synthesis, promotes platelet adhesion to endothelial cells [19].
Conjugated dienes, which are also products of LPO, have a damaging effect on proteins and lipoproteins [19]. The AOP system counteracts the activity of the LPO. In chronic diseases, especially in their comorbidity, the activity of AOP is reduced, resulting in the insufficient resistance to the damaging effects of the LPO [19].
SOD is a very important component of the AOP system. The antioxidant role of SOD is to bind ROS with the subsequent formation of hydrogen peroxide [19].
Not only lipids but also proteins of plasma membranes are subject to peroxidation under the action of ROS [9]. The interaction of ROS and protein molecules can lead to oxidative modification of amino acids — oxidation of the sulfhydryl group of cysteine, imidazole group of histidine, cyclic rings of tyrosine, phenylalanine and tryptophan [13].
The negative effect of oxidatively modified proteins is associated with the depletion of cellular antioxidants, due to the fact that oxidative proteins are a source of FR and the trigger of pathological processes under stress [9].
Free radicals cause oxidative DNA damage (about 100 variants of damage have been identified), which has been shown in vitro. The result is breaks in the polynucleotide chain of the molecule, modification of the carbohydrate moiety and nitrogenous bases. FRO of proteins can lead to various mutations [13, 27].
Protein peroxidation is the earliest marker of oxidative stress. The dynamics of changes in the products of protein peroxidation is a reflection of the degree of oxidative damage to cells and reserve-adaptation capabilities of the body [9].
Hydroxyl radicals attack monosaccharides, in particular glucose. As a result, oxidized monosaccharides are converted into dicarbonyl compounds and break a possible free radical chain, manifesting themselves as antioxidants [13, 28–30].
The AOP system in the body counteracts the negative effects of oxidative stress. There are various mechanisms of oxidative stress inhibition, which differ in structure and point of application in the chain of branched reactions of FRO processes [9].
The antioxidant system is divided into two parts: enzymatic and non-enzymatic. The activity of antioxidants is determined by stereoelectronic effects of aromatic and chroman rings, ortho- and paraposition of hydroxyl groups, thiol compounds, chelation of metals of variable valency, receptor interactions with the cell membrane, etc. [9, 31, 32].
Enzymatic antioxidants are highly effective. Hydrogen peroxide decomposes copper- and zinc-containing SOD, heme-containing catalase, selenium-containing glutathione peroxidase, which block the formation of a more aggressive hydroxyl radical. Exogenous natural and synthetic antioxidants are the main corrective factors in the depletion of the body’s enzyme defenses [9, 33].
Non-enzymatic antioxidants can be both lipophilic: tocopherol, vitamin A, ubiquinone, beta-carotenoids, and hydrophilic: ascorbic acid, lipoic acid, flavonoids, glutathione. Insufficient functioning of the antioxidant system can initiate the development of inflammation, hypersensitivity, and autoimmune reactions [9, 34].
All living organisms (except obligate anaerobes) have a number of hereditary, genetically determined, antioxidant mechanisms of detoxification of potentially dangerous ROS [1].
The multicomponent antioxidant system is represented by high- and low-molecular-weight compounds that exhibit specific and nonspecific antioxidant activity. The antioxidant system has mechanisms to eliminate oxidative damage, which are aimed at repairing, removing or replacing damaged molecules [1, 35].
Specific AOP is aimed at reducing and directly destroying ROS in tissues both enzymatically and non-enzymatically. The action of nonspecific AOP is aimed at preventing the possibility of additional generation of FR by eliminating the pool of metals of variable valency [1, 36].
Enzymatic components of the antioxidant system such as SOD, catalase, glutathione peroxidase, glutathione-S-transferase, glutathione reductase play an important role. The actions of antioxidant enzymes are closely related to each other and clearly balanced [1, 3].
SOD protects the body from highly toxic oxygen radicals. SOD is found in all oxygen-absorbing cells, it catalyzes the dismutation of superoxide to oxygen and hydrogen peroxide. The reaction rate is extremely high and is limited only by the rate of oxygen diffusion. The catalytic cycle of this enzyme includes the reduction and oxidation of the metal ion in the active center of the enzyme [9, 10, 37].
There are three forms of SOD: the first, containing copper, is in the cytosol, the second, containing zinc, is extracellular and the third, containing magnesium, is in the mitochondrial matrix. SOD causes inactivation of oxygen radicals that occur during electron transfer reactions or when exposed to metals with variable valency, ionizing, ultraviolet radiation, ultrasound, hyperbaric oxygenation, various diseases [9].
Overproduction of SOD causes decreased gene expression of one of the regulators of oxidative stress — SoxRS, which is activated by O2 [13].
SOD provides detoxification of the superoxide radical, and catalase — the destruction of hydrogen peroxide. It is known that the resistance of cells to the action of FR is due to these enzymes [9].
Glutathione peroxidase is also an important component of the enzyme unit of the AOP. In the peptide chain of glutathione peroxidаse, there is a residue of selenocysteine, a cysteine analogue in which the sulfur atom is replaced by a selenium atom [9].
The active center of the enzyme is selenocysteine. Glutathione peroxidase can reduce hydroperoxides of free fatty acids, hydroperoxides of phospholipids, esterified fatty acids [9].
Glutathione peroxidase is reduced by NADP-dependent enzyme glutathione reductase. Disulfide is formed during the oxidation of two molecules of the reduced form of glutathione [9].
The main antioxidant of erythrocytes is reduced glutathione, it is a coenzyme in the reduction of methemoglobin to functionally active hemoglobin. Detoxification of hydrogen peroxide and hydroperoxides, which are formed by the reaction of ROS with unsaturated fatty acids of the erythrocyte membrane, occurs with the help of reduced glutathione [9, 38, 39].
Inhibition of sodium-glucose cotransporter-2 and caloric restriction reduces the accumulation of oxidized glutathione in the renal cortex [40].
Intensive insulin therapy is able to normalize glutathione synthesis in stress-induced hyperglycaemia [41].
Catalase concentration is highest in the cells of the liver, kidneys, in erythrocytes. Catalase is formed by four identical subunits, each of which contains a prosthetic heme group. The iron atom in the heme is in the trivalent state [9].
For catalase of the human body, the optimal pH is 7 but in the range between pH 6.8 and 7.5, catalase activity does not change significantly. Catalase is one of the fastest enzymes, it does not lose activity for a long time, almost does not require activation energy, the reaction rate is limited only by the rate of diffusion of the substrate to the active site [9].
One molecule of catalase is able to convert several million molecules of hydrogen peroxide into water and oxygen per 1 second. Catalase degrades hydrogen peroxide, breaking the chain of conversion of superoxide anion into hydroxyl radical (O2 → H2O2 → OH•). Catalase, also in the presence of hydrogen peroxide, can oxidize various toxins such as formaldehyde, phenols, and alcohols. Competitive inhibitor is cyanide ion, non-competitive inhibitors of this enzyme are heavy metal ions [9, 10, 13].
The effectiveness of the body’s adaptive responses can be judged by assessing the state of the AОР system [9].
Recently, there is growing evidence for the important role of oxidative, nitrosative and carbonyl stresses in the development of chronic kidney disease (CKD) [1, 5, 42–45].
There are four main mechanisms of development of oxidative stress in the kidneys in diabetic nephropathy: direct inhibition of cellular antioxidant systems by glucose and its metabolites, accumulation of glycation end products, activation of protein kinase C, activation of renin-angiotensin-aldosterone system. NADPH oxidase is activated in the kidneys, which catalyse the formation of ROS [36, 46–49].
Activation of the antioxidant transcription factor Nrf2 in the renal tubules in mice with renal ischemic reperfusion injury effectively reduces tubular damage and interstitial fibrosis by inducing the expression of genes associated with cytoprotection against oxidative stress. Moreover, since the kidney performs several functions in addition to blood purification, renoprotection by Nrf2 activation is expected to lead to various benefits. Experiments have shown that the activation of Nrf2 alleviates anemia caused by impaired erythropoietin production, which is a factor in the growth of erythropoiesis, kidney damage, and reduces organ damage exacerbated by anemic hypoxia, which matched clinical water balance [50–57].
There is no doubt that oxidative stress adversely affects the kidneys and the course of CKD, which, in turn, itself contributes to the emergence and intensification of the damaging effects of oxidative stress.
Many questions about the effects of oxidative, nitrosative, and carbonyl stresses on renal status and the course of CKD remain unresolved. In this regard, it is important to seek new or improve diagnostic techniques using already known markers to assess the status of the kidneys and the course of CKD for timely and pathogenetically sound correction of kidney damage.
Received 01.12.2021
Revised 13.12.2021
Accepted 20.12.2021
Список литературы
1. Оксенюк О.С., Калмыкова Ю.А., Смирнова О.Б., Пасечник Д.Г. Роль окислительного стресса в развитии хронической болезни почек и способы его оценки. Журнал фундаментальной медицины и биологии. 2016. 1. Режим доступа: https://cyberleninka.ru/article/n/rol-okislitelnogo-stressa-v-razvitii-hronicheskoy-bolezni-pochek-i-sposoby-ego-otsenki.
2. Биохимия оксидативного стресса: учебно-методическое пособие. ФГБОУ ВО РНИМУ имени Н.И. Пирогова Минздрава России. Москва: Издательство ХХ, 2018.
3. Chu Y., Lan R.S., Huang R., Feng H., Kumar R., Dayal S., Chan K.S., Dai D.F. Glutathione peroxidase-1 overexpression reduces oxidative stress, and improves pathology and proteome remodeling in the kidneys of old mice. Aging Cell. 2020 Jun. 19(6). e13154. doi: 10.1111/acel.13154.
4. Stokman G., Kors L., Bakker P.J., Rampanelli E., Claessen N., Teske G.J.D., Butter L., van Andel H., van den Bergh Weerman M.A., Larsen P.W.B., Dessing M.C., Zuurbier C.J., Girardin S.E., Florquin S., Leemans J.C. NLRX1 dampens oxidative stress and apoptosis in tissue injury via control of mitochondrial activity. J. Exp. Med. 2017 Aug 7. 214(8). 2405-2420. doi: 10.1084/jem.20161031.
5. Seraphim D.C.C., Punaro G.R., Fernandes T.O., Ginoza M., Lopes G.S., Higa E.M.S. Assessment of fructose overload in the metabolic profile and oxidative/nitrosative stress in the kidney of senescent female rats. Exp. Gerontol. 2017 Dec 1. 99. 53-60. doi: 10.1016/j.exger.2017.09.011.
6. Csiszar A., Toth J., Peti-Peterdi J., Ungvari Z. The aging kidney: role of endothelial oxidative stress and inflammation. Acta Physiol. Hung. 2007 Mar. 94(1–2). 107-15. doi: 10.1556/APhysiol.94.2007.1-2.10.
7. Hirakawa Y., Inagi R. Glycative stress and its defense machinery glyoxalase 1 in renal pathogenesis. Int. J. Mol. Sci. 2017 Jan 17. 18(1). 174. doi: 10.3390/ijms18010174.
8. Melchioretto E.F., Zeni M., Veronez D.A.D.L., Filipak Neto F., Digner I.S., Fraga R. Stereological study and analysis of oxidative stress during renal aging in rats. Acta Cir. Bras. 2020 Dec 18. 35(11). e351106. doi: 10.1590/ACTA351106.
9. Нетюхайло Л.Г., Харченко С.В. Aктивні форми кисню (огляд літератури). Молодий вчений. 2014. 9(12). 131-135.
10. Kuma A., Mafune K., Uchino B. et al. Alteration of normal level of serum urate may contribute to decrease in estimated glomerular filtration rate decline in healthy Japanese men. Renal Failure. 2021 Dec. 43(1). 1408-1415. doi: 10.1080/0886022x.2021.1988969.
11. Остапченко Л.І., Компанець І.В., Скопенко О.В. та ін. Біохімія. Практикум. Київ: Видавничо-поліграфічний центр «Київський університет», 2018.
12. Оксидативный стресс и воспаление: патогенетическое партнерство: Монография. Под ред. О.Г. Хурцилавы, Н.Н. Плужникова, Я.А. Накатиса. Санкт-Петербург: Издательство СЗГМУ им. И.И. Мечникова, 2012. 340 с.
13. Семчишин Г.М., Лущак В.І. Oксидативний стрес і регуляція активності каталаз у Escherichia coli. Укр. біохім. журн. 2004. 76(2). 31-42.
14. Ghosh M., Das J., Sil P.C. D(+) galactosamine induced oxidative and nitrosative stress-mediated renal damage in rats via NF-κB and inducible nitric oxide synthase (iNOS) pathways is ameliorated by a polyphenol xanthone, mangiferin. Free Radic. Res. 2012 Feb. 46(2). 116-32. doi: 10.3109/10715762.2011.644240.
15. Han Q., Zhang J., Sun Q., Xu Y., Teng X. Oxidative stress and mitochondrial dysfunction involved in ammonia-induced nephrocyte necroptosis in chickens. Ecotoxicol. Environ. Saf. 2020 Oct 15. 203. 110974. doi: 10.1016/j.ecoenv.2020.110974.
16. Mattace Raso G., Simeoli R., Russo R., Santoro A., Pirozzi C., d’Emmanuele di Villa Bianca R., Mitidieri E., Paciello O., Pagano T.B., Orefice N.S., Meli R., Calignano A. N-palmitoylethanolamide protects the kidney from hypertensive injury in spontaneously hypertensive rats via inhibition of oxidative stress. Pharmacol. Res. 2013 Oct. 76. 67-76. doi: 10.1016/j.phrs.2013.07.007.
17. Aoki K., Yanazawa K., Tokinoya K., Sugasawa T., Suzuki T., Yoshida Y., Nakano T., Omi N., Kawakami Y., Takekoshi K. Renalase is localized to the small intestine crypt and expressed upon the activation of NF-κB p65 in mice model of fasting-induced oxidative stress. Life Sci. 2021 Feb 15. 267. 118904. doi: 10.1016/j.lfs.2020.118904.
18. Zhang F., Lu Z., Wang F. Advances in the pathogenesis and prevention of contrast-induced nephropathy. Life Sci. 2020 Oct 15. 259. 118379. doi: 10.1016/j.lfs.2020.118379.
19. Просоленко К.О. Показники оксидативного стресу та антиоксидантної активності при коморбідності неалкогольної жирової хвороби печінки та артеріальної гіпертензії. Український журнал медицини, біології та спорту. 2020. 5. 1(23). 179-186. doi: 10.26693/jmbs05.01.179.
20. Rabbani N., Thornalley P.J. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018 Apr. 93(4). 803-813. doi: 10.1016/j.kint.2017.11.034.
21. Ara C., Dirican A., Unal B., Bay Karabulut A., Piskin T. The effect of melatonin against FK506-induced renal oxidative stress in rats. Surg. Innov. 2011 Mar. 18(1). 34-8. doi: 10.1177/1553350610381088.
22. Suzuki D., Miyata T. Carbonyl stress in the pathogenesis of diabetic nephropathy. Intern. Med. 1999 Apr. 38(4). 309-14. doi: 10.2169/internalmedicine.38.309.
23. Fatih Aydın A., Küçükgergin C., Bingül İ., Doğan-Ekici I., Doğru-Abbasoğlu S., Uysal M. Effect of carnosine on renal function, oxidation and glycation products in the kidneys of high-fat diet/streptozotocin-induced diabetic rats. Exp. Clin. Endocrinol. Diabetes. 2017 May. 125(5). 282-289. doi: 10.1055/s-0043-100117.
24. Samarghandian S., Azimi-Nezhad M., Farkhondeh T., Samini F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed. Pharmacother. 2017 Mar. 87. 223-229. doi: 10.1016/j.biopha.2016.12.105.
25. Dar M.A., Khan A.M., Raina R., Verma P.K., Wani N.M. Effect of bifenthrin on oxidative stress parameters in the liver, kidneys, and lungs of rats. Environ. Sci. Pollut. Res. Int. 2019 Mar. 26(9). 9365-9370. doi: 10.1007/s11356-019-04362-4.
26. Farías J.G., Zepeda A.B., Calaf G.M. Melatonin protects the heart, lungs and kidneys from oxidative stress under intermittent hypobaric hypoxia in rats. Biol. Res. 2012. 45(1). 81-5. doi: 10.4067/S0716-97602012000100011.
27. Othmène Y.B., Hamdi H., Salem I.B., Annabi E., Amara I., Neffati F., Najjar M.F., Abid-Essefi S. Oxidative stress, DNA damage and apoptosis induced by tebuconazole in the kidney of male Wistar rat. Chem. Biol. Interact. 2020 Oct 1. 330. 109114. doi: 10.1016/j.cbi.2020.109114.
28. Song Y.R., Kim J.K., Lee H.S., Kim S.G., Choi E.K. Serum levels of protein carbonyl, a marker of oxidative stress, are associated with overhydration, sarcopenia and mortality in hemodialysis patients. BMC Nephrol. 2020 Jul 16. 21(1). 281. doi: 10.1186/s12882-020-01937-z.
29. Caimi G., Hopps E., Montana M., Carollo C., Calandrino V., Gallà E., Canino B., Lo Presti R. Behaviour of carbonyl groups in several clinical conditions: analysis of our survey. Clin. Hemorheol. Microcirc. 2020. 74(3). 299-313. doi: 10.3233/CH-190689.
30. Colombo G., Reggiani F., Angelini C., Finazzi S., Astori E., Garavaglia M.L., Landoni L., Portinaro N.M., Giustarini D., Rossi R., Santucci A., Milzani A., Badalamenti S., Dalle-Donne I. Plasma protein carbonyls as biomarkers of oxidative stress in chronic kidney disease, dialysis, and transplantation. Oxid. Med. Cell. Longev. 2020 Nov 24. 2020. 2975256. doi: 10.1155/2020/2975256.
31. Mukhopadhyay P., Pan H., Rajesh M., Bátkai S., Patel V., Harvey-White J., Mukhopadhyay B., Haskó G., Gao B., Mackie K., Pacher P. CB1 cannabinoid receptors promote oxidative/nitrosative stress, inflammation and cell death in a murine nephropathy model. Br. J. Pharmacol. 2010 Jun. 160(3). 657-68. doi: 10.1111/j.1476-5381.2010.00769.x.
32. Jia Y., Wang L., Zhao G.Y., Wang Z.Q., Chen S., Chen G. Carbon monoxide inhibits the nuclear-cytoplasmic translocation of HMGB1 in an in vitro oxidative stress injury model of mouse renal tubular epithelial cells. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2016 Dec. 36(6). 791-795. doi: 10.1007/s11596-016-1663-y.
33. Kumar A., Hammad A., Sharma A.K., Mc-Cardle F., Rustom R., Christmas S.E. Oxidative stress in kidney transplant biopsies. Exp. Clin. Transplant. 2015 Apr. 13 Suppl. 1. 207-13.
34. Guzmán-Guillén R., Prieto A.I., Vázquez C.M., Vasconcelos V., Cameán A.M. The protective role of l-carnitine against cylindrospermopsin-induced oxidative stress in tilapia (Oreochromis niloticus). Aquat. Toxicol. 2013 May 15. 132-133. 141-50. doi: 10.1016/j.aquatox.2013.02.011.
35. Yan J., Wang D., Miao J., Liu C., Wang Y., Teng M., Zhou Z., Zhu W. Discrepant effects of α-endosulfan, β-endosulfan, and endosulfan sulfate on oxidative stress and energy metabolism in the livers and kidneys of mice. Chemosphere. 2018 Aug. 205. 223-233. doi: 10.1016/j.chemosphere.2018.04.101.
36. Ishimoto Y., Tanaka T., Yoshida Y., Inagi R. Physiological and pathophysiological role of reactive oxygen species and reactive nitrogen species in the kidney. Clin. Exp. Pharmacol. Physiol. 2018 Nov. 45(11). 1097-1105. doi: 10.1111/1440-1681.13018.
37. Santos E.B., Koff W.J., Grezzana Filho T. de J.M., De Rossi S.D., Treis L., Bona S.R., Pêgas K.L., Katz B., Meyer F.S., Marroni N.A., Corso C.O. Oxidative stress evaluation of ischemia and reperfusion in kidneys under various degrees of hypothermia in rats. Acta Cir. Bras. 2013 Aug. 28(8). 568-73. doi: 10.1590/s0102-86502013000800003.
38. Hua W., Huang H.Z., Tan L.T., Wan J.M., Gui H.B., Zhao L., Ruan X.Z., Chen X.M., Du X.G. CD36 mediated fatty acid-induced podocyte apoptosis via oxidative stress. PLoS One. 2015 May 22. 10(5). e0127507. doi: 10.1371/journal.pone.0127507.
39. Ossani G.P., Uceda A.M., Acosta J.M., Lago N.R., Repetto M.G., Martino D.J., Toblli J.E. Role of oxidative stress in lithium-induced nephropathy. Biol. Trace Elem. Res. 2019 Oct. 191(2). 412-418. doi: 10.1007/s12011-018-1617-2.
40. Tanaka S., Sugiura Y., Saito H., Sugahara M., Higashijima Y., Yamaguchi J., Inagi R., Suematsu M., Nangaku M., Tanaka T. Sodium-glucose cotransporter 2 inhibition normalizes glucose metabolism and suppresses oxidative stress in the kidneys of diabetic mice. Kidney Int. 2018 Nov. 94(5). 912-925. doi: 10.1016/j.kint.2018.04.025.
41. Добреля Н.В., Хромов О.С. Цукровий діабет та мале коло кровообігу (частина 1). Фізіол. журн. 2019. 65(2). 97-107.
42. Hara M., Torisu K., Tomita K., Kawai Y., Tsuruya K., Nakano T., Kitazono T. Arginase 2 is a mediator of ischemia-reperfusion injury in the kidney through regulation of nitrosative stress. Kidney Int. 2020 Sep. 98(3). 673-685. doi: 10.1016/j.kint.2020.03.032.
43. Punaro G.R., Lima D.Y., Rodrigues A.M., Pugliero S., Mouro M.G., Rogero M.M., Higa E.M.S. Cupuaçu extract reduces nitrosative stress and modulates inflammatory mediators in the kidneys of experimental diabetes. Clin. Nutr. 2019 Feb. 38(1). 364-371. doi: 10.1016/j.clnu.2017.12.016.
44. Pessoa E.A., Convento M.B., Castino B., Leme A.M., de Oliveira A.S., Aragão A., Fernandes S.M., Carbonel A., Dezoti C., Vattimo M.F., Schor N., Borges F.T. Beneficial effects of isoflavones in the kidney of obese rats are mediated by PPAR-gamma expression. Nutrients. 2020 Jun 1. 12(6). 1624. doi: 10.3390/nu12061624.
45. Sheehan D., Rainville L.C., Tyther R., McDonagh B. Redox proteomics in study of kidney-associated hypertension: new insights to old diseases. Antioxid. Redox Signal. 2012 Dec 1. 17(11). 1560-70. doi: 10.1089/ars.2012.4705.
46. Mohebbati R., Abbasnezhad A., Havakhah S., Mousavi M. The effect of Nigella sativa on renal oxidative injury in diabetic rats. Saudi J. Kidney Dis. Transpl. 2020 Jul-Aug. 31(4). 775-786. doi: 10.4103/1319-2442.292311.
47. Arany I., Hall S., Reed D.K., Reed C.T., Dixit M. Nicotine enhances high-fat diet-induced oxidative stress in the kidney. Nicotine Tob. Res. 2016 Jul. 18(7). 1628-34. doi: 10.1093/ntr/ntw029.
48. Ueno Y., Horio F., Uchida K., Naito M., Nomura H., Kato Y., Tsuda T., Toyokuni S., Osawa T. Increase in oxidative stress in kidneys of diabetic Akita mice. Biosci. Biotechnol. Biochem. 2002 Apr. 66(4). 869-72. doi: 10.1271/bbb.66.869.
49. Жариков А.Ю., Филинова С.О., Мазко О.Н., Макарова О.Г., Бобров И.П., Брюханов В.М. Роль свободнорадикального окисления в почках в нефропротекторном действии блокатора минералокортикоидных рецепторов эплеренона при экспериментальном сахарном диабете. Бюллетень сибирской медицины. 2021. 2. Режим доступа: https://cyberleninka.ru/article/n/rol-svobodnoradikalnogo-okisleniya-v-pochkah-v-nefroprotektornom-deystvii-blokatora-mineralokortikoidnyh-retseptorov-eplerenona-pri.
50. Nezu M., Suzuki N. Roles of Nrf2 in protecting the kidney from oxidative damage. Int. J. Mol. Sci. 2020 Apr 22. 21(8). 2951. doi: 10.3390/ijms21082951.
51. Su H., Wan C., Song A., Qiu Y., Xiong W., Zhang C. Oxidative stress and renal fibrosis: mechanisms and therapies. Adv. Exp. Med. Biol. 2019. 1165. 585-604. doi: 10.1007/978-981-13-8871-2_29.
52. Stryjak I., Warmuzińska N., Bogusiewicz J., Łuczykowski K., Bojko B. Monitoring of the influence of long-term oxidative stress and ischemia on the condition of kidneys using solid-phase microextraction chemical biopsy coupled with liquid chromatography-high-resolution mass spectrometry. J. Sep. Sci. 2020 May. 43(9–10). 1867-1878. doi: 10.1002/jssc.202000032.
53. Zhang Y., Wang J., Liu X., Ding L., Wu X., He M., Hou H., Ruan G., Lai J., Chen C. An investigation into the effects of long-term 50-Hz power-frequency electromagnetic field exposure on hematogram, blood chemistry, fibrosis, and oxidant stress status in the liver and the kidney from Sprague-Dawley rats. Bioelectromagnetics. 2020 Oct. 41(7). 511-525. doi: 10.1002/bem.22291.
54. Martínez-Lara E., Peña A., Calahorra J., Cañuelo A., Siles E. Hydroxytyrosol decreases the oxidative and nitrosative stress levels and promotes angiogenesis through HIF-1 independent mechanisms in renal hypoxic cells. Food Funct. 2016 Jan. 7(1). 540-8. doi: 10.1039/c5fo00928f.
55. Caliskan B., Guven A., Ozler M., Cayci T., Ozcan A., Bedir O., Surer I., Korkmaz A. Ozone therapy prevents renal inflammation and fibrosis in a rat model of acute pyelonephritis. Scand. J. Clin. Lab. Invest. 2011 Oct. 71(6). 473-80. doi: 10.3109/00365513.2011.587022.
56. Mendonca P., Soliman K.F.A. Flavonoids activation of the transcription factor Nrf2 as a hypothesis approach for the prevention and modulation of SARS-CoV-2 infection severity. Antioxidants (Basel). 2020 Jul 24. 9(8). 659. doi: 10.3390/antiox9080659.
57. Ivanova M.D., Gozhenko A.I., Crestanello T., Dmytro D. Ivanov. Early coaching to increase water intake in CKD. Ann. Nutr. Metab. 2021. doi: 10.1159/000515276.

/58.jpg)
/59.jpg)
/60.jpg)