Журнал «Практическая онкология» Том 6, №1, 2023
Вернуться к номеру
Пухлиноінфільтруючі лімфоцити: нові виклики імунотерапії солідних пухлин
Авторы: Ніколаєва О.Ю.
Національний медичний університет ім. О.О. Богомольця, м. Київ, Україна
Рубрики: Онкология
Разделы: Справочник специалиста
Версия для печати
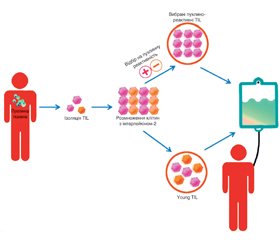
Пухлиноінфільтруючі лімфоцити (tumor infiltrative lymphocytes, TIL) — це лімфоцити, що локалізуються у пухлинній тканині. Після ізоляції, скринінгу та ампліфікації in vitro вони вводяться назад в організм пацієнта і мають специфічний знищуючий ефект на пухлини. Оскільки TIL не були генетично модифіковані та походять з організму пацієнтів, побічних реакцій зазвичай виникає відносно мало, що є перевагою лікування за допомогою TIL. Останніми роками терапевтична дія TIL на солідні пухлини почала привертати все більше уваги онкологів. Однак через обмеження імунного мікрооточення та мутації антигенів розвиток TIL-терапії сповільнився. У статті розглядається загальний прогрес дослідження TIL, біологічні характеристики, методи посилення терапевтичного ефекту пухлиноінфільтруючих лімфоцитів, їх роль у різних пухлинах, дані останніх клінічних досліджень та перспективи цього виду терапії.
Tumor-infiltrating lymphocytes (TIL) are lymphocytes localized in tumor tissue. After isolation, screening and amplifying ex vivo, they are injected back into the patient’s body and have a specific tumor-killing effect. Because TIL have not been genetically modified and come from the patient’s body, there are usually relatively few adverse reactions, which is an advantage of TIL treatment. In recent years, the therapeutic effect of TIL on solid tumors has begun to attract more and more attention of oncologists. However, the development of TIL therapy has slowed down due to limitations of the immune microenvironment and antigen mutations. This article considers the overall progress in TIL investigation, biological characteristics, methods of enhancing the therapeutic effect of tumor-infiltrating lymphocytes, their role in various tumors, current achievements in clinical trials, and general perspectives of this type of therapy.
пухлиноінфільтруючі лімфоцити; імунотерапія; пухлина; солідні пухлини
tumor-infiltrating lymphocytes; immunotherapy; tumor; solid tumors
Для ознакомления с полным содержанием статьи необходимо оформить подписку на журнал.
- Darvin P., Toor S.M., Sasidharan Nair V., Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp. Mol. Med. 2018. 50.
- Balkwill F.R., Capasso M., Hagemann T. The tumor microenvironment at a glance. J. Cell Sci. 2012. 125. 5591-5596.
- Paijens S.T., Vledder A., de Bruyn M., Nijman H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cellular and Molecular Immunology. 2021. 18. 842-859.
- Valkenburg K.C., de Groot A.E., Pienta K.J. Targeting the tumor stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018. 15. 366-381.
- Bazzichetto C., Conciatori F., Falcone I. et al. Advances in Tumor-Stroma Interactions. Emerging Role of Cytokine Network in Colorectal and Pancreatic Cancer. J. Oncol. 2019. 2019.
- di Caro G., Bergomas F., Grizzi F. et al. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin. Cancer Res. 2014. 20. 2147-2158.
- Fridman W.H., Pagès F., Saut̀s-Fridman C., Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer. 2012. 12. 298-306.
- Tsakiroglou A.M., Fergie M., Oguejiofor K. et al. Spatial proximity between T and PD-L1 expressing cells as a prognostic biomarker for oropharyngeal squamous cell carcinoma. Br. J. Cancer. 2020. 122. 539-544.
- Bouzin C., Brouet A., de Vriese J., DeWever J., Feron O. Effects of vascular endothelial growth factor on the lymphocyte-endothelium interactions: identification of caveolin-1 and nitric oxide as control points of endothelial cell anergy. J. Immunol. 2007. 178. 1505-1511.
- Buckanovich R.J., Facciabene A., Kim S. et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat. Med. 2008. 14. 28-36.
- Shrimali R.K., Yu Z., Theoret M.R., Chinnasamy D., Restifo N.P., Rosenberg S.A. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010. 70. 6171-6180.
- Rosenberg S.A., Packard B.S., Aebersold P.M. et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunothe–rapy of patients with metastatic melanoma. A preliminary report. N. Engl. J. Med. 1988. 319. 1676-1680.
- Huang J., Khong H.T., Dudley M.E. et al. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J. Immunother. 2005. 28. 258-267.
- Powell D.J., Dudley M.E., Robbins P.F., Rosenberg S.A. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005. 105. 241-250.
- Wu R., Forget M.A., Chacon J. et al. Adoptive T-cell the–rapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer J. 2012. 18. 160-175.
- Tran K.Q., Zhou J., Durflinger K.H. et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J. Immunother. 2008. 31. 742-751.
- Hall M.L., Liu H., Malafa M. et al. Expansion of tumor-infiltrating lymphocytes (TIL) from human pancreatic tumors. J. Immunother. Cancer. 2016. 4.
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011. 144. 646-674.
- Vogelstein B., Papadopoulos N., Velculescu V.E., Zhou S., Diaz L.A., Kinzler K.W. Cancer genome landscapes. Science. 2013. 339. 1546-1558.
- Galluzzi L., Chan T.A., Kroemer G., Wolchok J.D., López-Soto A. The hallmarks of successful anticancer immunotherapy. Sci. Transl. Med. 2018. 10.
- Majzner R.G., Mackall C.L. Clinical lessons learned from the first leg of the CAR T cell journey. Nat. Med. 2019. 25. 1341-1355.
- Sharma P., Hu-Lieskovan S., Wargo J.A., Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017. 168. 707-723.
- Anderson K.G., Stromnes I.M., Greenberg P.D. Obstacles Posed by the Tumor Microenvironment to T cell Activity. A Case for Synergistic Therapies. Cancer. Cell. 2017. 31. 311-325.
- Schumacher T.N., Schreiber R.D. Neoantigens in cancer immunotherapy. Science. 2015. 348. 69-74.
- Titov A., Zmievskaya E., Ganeeva I. et al. Adoptive Immunotherapy beyond CAR T-Cells. Cancers (Basel). 2021. 13. 1-23.
- Bedognetti D., Spivey T.L., Zhao Y. et al. CXCR3/CCR5 pathways in metastatic melanoma patients treated with adoptive the–rapy and interleukin-2. Br. J. Cancer. 2013. 109. 2412-2423.
- Mikucki M.E., Fisher D.T., Matsuzaki J. et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat. Commun. 2015. 6.
- van Kooyk Y., van de Wiel-Van Kemenade P., Weder P., Kuijpers T.W., Figdor C.G. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature. 1989. 342. 811-813.
- Burbach B.J., Medeiros R.B., Mueller K.L., Shimizu Y. –T-cell receptor signaling to integrins. Immunol. Rev. 2007. 218. –65-81.
- Mirenda V., Jarmin S.J., David R. et al. Physiologic and aberrant regulation of memory T-cell trafficking by the costimulatory molecule CD28. Blood. 2007. 109. 2968-2977.
- Cameron B.J., Gerry A.B., Dukes J. et al. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci. Transl. Med. 2013. 5.
- Morgan R.A., Chinnasamy N., Abate-Daga D. et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 2013. 36. 133-151.
- Paijens S.T., Vledder A., Loiero D. et al. Prognostic image-based quantification of CD8CD103 T cell subsets in high-grade serous ovarian cancer patients. Oncoimmunology. 2021. 10.
- Kim Y., Shin Y., Kang G.H. Prognostic significance of CD103+ immune cells in solid tumor: a systemic review and meta-analysis. Sci. Rep. 2019. 9.
- Bösmüller H.C., Wagner P., Peper J.K. et al. Combined immunoscore of CD103 and CD3 identifies long-term survivors in high-grade serous ovarian cancer. International Journal of Gynecological. Cancer. 2016. 26. 671-679.
- Mann J.E., Smith J.D., Birkeland A.C. et al. Analysis of tumor-infiltrating CD103 resident memory T-cell content in recurrent laryngeal squamous cell carcinoma. Cancer Immunology, Immunotherapy. 2019. 68. 213-220.
- Wang B., Wu S., Zeng H. et al. CD103+ Tumor Infiltrating Lymphocytes Predict a Favorable Prognosis in Urothelial Cell Carcinoma of the Bladder. Journal of Urology. 2015. 194. 556-562.
- Zhong J., Qin Y., Yu P. et al. The Landscape of the Tumor-Infiltrating Immune Cell and Prognostic Nomogram in Colorectal Cancer. Front Genet. 2022. 13. 891270.
- Solomon B., Young R.J., Bressel M. et al. Identification of an excellent prognosis subset of human papillomavirus-associated oropharyngeal cancer patients by quantification of intratumoral CD103+ immune cell abundance. Ann. Oncol. 2019. 30. 1638-1646.
- Hewavisenti R., Ferguson A., Wang K. et al. CD103+ tumor-resident CD8+ T cell numbers underlie improved patient survival in oropharyngeal squamous cell carcinoma. J. Immunother. Cancer. 2020. 8.
- Hu W., Sun R., Chen L., Zheng X., Jiang J. Prognostic significance of resident CD103 + CD8 + T cells in human colorectal cancer tissues. Acta Histochem. 2019. 121. 657-663.
- Edwards J., Wilmott J.S., Madore J. et al. CD103 + Tumor-Resident CD8 + T Cells Are Associated with Improved Survival in Immunotherapy-Naïve Melanoma Patients and Expand Significantly During Anti-PD-1 Treatment. Clin. Cancer Res. 2018. 24. 3036-3045.
- Han L., Gao Q.L., Zhou X.M. et al. Characterization of CD103+ CD8+ tissue-resident T cells in esophageal squamous cell carcinoma: may be tumor reactive and resurrected by anti-PD-1 blockade. Cancer Immunology, Immunotherapy. 2020. 69. 1493-1504.
- Corgnac S., Malenica I., Mezquita L. et al. CD103+CD8+ TRM Cells Accumulate in Tumors of Anti-PD-1-Responder Lung Cancer Patients and Are Tumor-Reactive Lymphocytes Enriched with Tc17. Cell Rep. Med. 2020. 1.
- Ahmadvand S., Faghih Z., Montazer M. et al. Importance of CD45RO+ tumor-infiltrating lymphocytes in post-operative survival of breast cancer patients. Cell Oncol. (Dordr.). 2019. 42. 343-356.
- Hwang C., Lee S.J., Lee J.H. et al. Stromal tumor-infiltra–ting lymphocytes evaluated on H&E-stained slides are an independent prognostic factor in epithelial ovarian cancer and ovarian serous carcinoma. Oncol. Lett. 2019. 17. 4557-4565.
- James F.R., Jiminez-Linan M., Alsop J. et al. Association between tumour infiltrating lymphocytes, histotype and clinical outcome in epithelial ovarian cancer. BMC Cancer. 2017. 17.
- Salgado R., Denkert C., Campbell C. et al. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab. A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol. 2015. 1. 448-455.
- Luen S.J., Salgado R., Fox S. et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. Lancet Oncol. 2017. 18. 52-62.
- Denkert C., von Minckwitz G., Brase J.C. et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. Journal of Clinical Oncology. 2015. 33. 983-991.
- Kim R.S., Song N., Gavin P.G. et al. Stromal Tumor-infiltrating Lymphocytes in NRG Oncology/NSABP B-31 Adjuvant Trial for Early-Stage HER2-Positive Breast Cancer. J. Natl. Cancer Inst. 2019. 111. 867-871.
- Fuchs T.L., Sioson L., Sheen A. et al. Assessment of Tumor-infiltrating Lymphocytes Using International TIL Working Group (ITWG) System Is a Strong Predictor of Overall Survival in Colorectal Carcinoma. A Study of 1034 Patients. Am. J. Surg. Pathol. 2020. 44.
- Treatment with tumor-infiltrating lymphocytes (TIL) versus ipilimumab for advanced melanoma. Results from a multicenter, randomized phase III trial. OncologyPRO. https://oncologypro.esmo.org/meeting-resources/esmo-congress/treatment-with-tumor-infiltrating-lymphocytes-til-versus-ipilimumab-for-advanced-me–lanoma-results-from-a-multicenter-randomized-phase-iii-trial (27 September 2022, date last accessed).
- Tran G.T., Hodgkinson S.J., Carter N. et al. Autoantigen specific IL-2 activated CD4 + CD25 + T regulatory cells inhibit induction of experimental autoimmune neuritis. J. Neuroimmunol. 2020. 341.
- Conlon K.C., Miljkovic M.D., Waldmann T.A. Cytokines in the Treatment of Cancer. J. Interferon Cytokine Res. 2019. 39. 6-21.
- Lowery F.J., Krishna S., Yossef R. et al. Molecular signatures of antitumor neoantigen-reactive T cells from metastatic human cancers. Science. 2022. 375. 877-884.
- Robertson J., Salm M., Dangl M. Adoptive cell therapy with tumour-infiltrating lymphocytes: the emerging importance of clonal neoantigen targets for next-generation products in non-small cell lung cancer. Immuno-Oncology Technology. 2019. 3. 1-7.
- Kim S.P., Vale N.R., Zacharakis N. et al. Adoptive Cellular Therapy with Autologous Tumor-Infiltrating Lymphocytes and T-cell Receptor-Engineered T Cells Targeting Common p53 Neoantigens in Human Solid Tumors. Cancer Immunol. Res. 2022. 10. 932-946.