Резюме
Актуальність. Метформін — один із цукрознижувальних засобів першої лінії, що найчастіше призначається для лікування хворих на цукровий діабет 2-го типу (ЦД2). Однак механізм його лікувальної дії ще недостатньо вивчений. У той же час ЦД2 вважається захворюванням запальної природи, при якому порушені різні імунні реакції. Однак вивченню ролі імунної системи в механізмі цього захворювання присвячені лише одиничні роботи. Мета дослідження: висвітлення питання про те, якою мірою різні види лейкоцитів, імунофенотип лімфоцитів та низка цитокінів беруть участь у механізмі терапевтичної дії метформіну. Матеріали та методи. Обстежені група хворих обох статей з уперше виявленим ЦД2 з індексом маси тіла 33,1 ± 1,3 кг/м2, яких ще не лікували цукрознижувальними засобами, та група нормоглікемічних здорових осіб того ж віку та статі. Кількість лейкоцитів у периферичній крові (ПК) визначали
за допомогою гематологічних аналізаторів, а лейкоцитарний склад — у мазках, пофарбованих за Папенгеймом. Імунофенотип лімфоцитів (CD3+ T, CD4+ T, CD8+ T,
CD56+) визначали методом проточної цитометрії за допомогою цитофлуориметра FACStar plus. Вміст різних цитокінів (ІЛ-1β, ФНП-α та ІЛ-10) — імуноферментним методом ELISA. Результати. Терапія метформіном хворих з уперше виявленим ЦД2 та ознаками ожиріння приводить до нормалізації підвищеної кількості лейкоцитів, нейтрофілів та моноцитів, а також зниження
вмісту CD4+Т клітин у ПК, особливо у хворих із високими показниками індексу маси тіла. Характерною особливістю терапії є різке зниження рівня прозапальних цитокінів (ІЛ-1β та ФНП-α), підвищеного до лікування. Отримані дані вказують на те, що при ЦД2 відзначаються порушення природженого й адаптивного імунітету, та підтверджують гіпотезу про запальну природу цього захворювання. Висновки. Сприятлива лікувальна дія метформіну при ЦД2, особливо ускладненого ожирінням, багато в чому зумовлена нормалізацією показників запалення та імунітету.
Актуальность. Метформин является одним из сахароснижающих препаратов первой линии, наиболее часто назначаемым для лечения больным сахарных диабетом 2-го типа (СД2). Однако механизм его лечебного действия еще недостаточно изучен. В то же время СД2 считается заболеванием воспалительной природы, при котором нарушены различные иммунные реакции. Однако роли иммунной системы в механизме лечебного действия метформина посвящены лишь единичные работы. Цель исследования: выяснение вопроса о том, в какой мере различные виды лейкоцитов, иммунофенотип лимфоцитов и ряд цитокинов участвуют в механизме терапевтического действия метформина. Материалы и методы. Обследована группа больных обоих полов с впервые выявленным СД2 с индексом массы тела 33,1 ± 1,3 кг/м2, еще не принимавших сахароснижающих средств, и группа нормогликемических здоровых лиц того же возраста и пола. Количество лейкоцитов в периферической крови (ПК) определяли с помощью гематологических анализаторов, а лейкоцитарный состав — в мазках, окрашенных по Паппенгейму. Иммунофенотип лимфоцитов (CD3+ T, CD4+ T, CD8+ T,
CD56+) определялся методом проточной цитометрии с помощью цитофлуориметра FACStar plus. Содержание различных цитокинов (ИЛ-1β, ФНО-α и ИЛ-10) — иммуноферментным методом ELISA. Результаты. Терапия метформином впервые выявленных больных СД2 с признаками ожирения приводит к нормализации повышенного количества лейкоцитов, нейтрофилов и моноцитов, а также к снижению содержания CD4+ Т клеток в ПК, особенно у больных с высоким показателем индекса массы тела. Характерной особенностью терапии является резкое снижение уровня противовоспалительных цитокинов (ИЛ-1β и ФНО-α), повышенного до лечения. Полученные данные указывают на то, что при СД2 имеются значительные нарушения врожденного и адаптивного иммунитета, и подтверждают гипотезу о воспалительной природе этого заболевания. Выводы. Благоприятное лечебное действие метформина при СД2, особенно осложненном ожирением, во многом обусловлено нормализацией показателей воспаления и иммунитета.
Background. Metformin is one of the most prescribed hypoglycemic drugs of the first line of treatment for patients with type 2 diabetes (T2D). However, the mechanism of its therapeutic effect has not been sufficiently studied. At the same time, T2D is considered a disease of an inflammatory nature, wherein different immune responses are disturbed. However, only single articles are devoted to the role of the immune system in the mechanism of the therapeutic action of metformin. Purpose of the study. Elucidation of the question to what extent different types of leukocytes, immunophenotype of lymphocytes and some cytokines are involved in the mechanism of the therapeutic action of metformin. Materials and methods. A group of patients of both sexes with a newly diagnosed T2D with BMI of 33.1 ± 1.3 kg/m2 who had not yet taken hypoglycemic agents and a group of normoglycemic healthy individuals of the same age and sex were examined. The number of leukocytes in blood was determined using the hematological analyzers, and the leukocyte composition — in Pappenheim stained smears. Immunophenotype of lymphocytes (CD3+ T, CD4+ T, CD8+ T, CD56+) — by flow cytometry using the FACStar plus cytofluorimeter. The content of different cytokines (I-1β, TNF-α and IL-10) — with immunosorbent ELISA assay. Results. The metformin therapy of newly diagnosed T2D patients with obesity leads to normalization of the increased number of leukocytes, neutrophils and monocytes, as well as a decrease in CD4+ T cells in the blood, especially in patients with high BMI. A characteristic feature of the therapy is a sharp decrease in anti-inflammatory cytokines (IL-1β and TNF-α) that were elevated before treatment. The obtained data indicate significant disorders of natural and adaptive immunity in T2D and confirm the hypothesis about the inflammatory nature of this disease. Conclusions. The favorable therapeutic effect of metformin in T2D, especially complicated by obesity, is largely due to the normalization of inflammation and immunity indices
Metformin (1.1 dimethylbiguanide) is currently one of the most prescribed oral hypoglycemic effective drugs of the first line treatment for patients with type 2 diabetes (T2D) all over the world [19, 31]. There are also data on its use in prediabetes to prevent the development of T2D [37]. Metformin has a pluripotent effect, is not metabolized in vivo and has a 50 % bioavailability [14]. A particularly beneficial therapeutic effect of metformin is described in obese patients with T2D [29], cardiovascular complications [1, 24], and atherosclerosis [38]. It also has a prophylactic effect, preventing the transition of insulin resistance (IR) to clinically diagnosed T2D [25]. There is also evidence that metformin has an antitumor property [29].
Although metformin as a chemical compound was described about 100 years ago, its hypoglycemic prope–rties and safety of use in humans became known only after 50 years, its wide use for the treatment of diabetes practically began only from the last decade of the last century [31].
Until recently, the therapeutic properties of metformin were determined by its property to reduce the formation of glucose by the liver, and its absorption in the intestinal tract, improving the utilization of glucose by tissues, lowering the level of total cholesterol, low-density lipopolysaccharides and triglycerides.
However, in recent years, new unexpected data on the mechanism of the antihyperglycemic effect of metformin in T2D have been obtained, which require the correction in the essential “classical” representations on this issue. Thus, in the study of patients with metformin-treated T2D, it was found [5, 31] that the initial primary hypoglycemic effect of metformin is not carried out in circulation (blood plasma), but in the duodenum and small intestine where L and K cells secrete the incretin hormone — glucagon-like peptide-1 (GLP-1) [21, 28]. It should be emphasized that the accumulation of glucose in the intestine after the administration of metformin is 300 times greater than in the blood plasma, while the accumulation of glucose in the liver is only 10 times greater than in the circulating blood [5].
At the same time, there were reports of a favorable therapeutic effect of the association of metformin and GLP-1 preparations [26], and that of a certain role of microbiota (a set of microbes in the intestine) [9].
As is known, numerous diverse studies have been devoted to elucidating the mechanism of the therapeutic action of metformin in T2D, but the information of the immune system participation in it are extremely limited and are presented only in a few fragmentary ambiguous publications [6, 16, 22, 40]. At the same time, accor–ding to modern ideas, T2D in humans is an inflammatory disease in the pathogenesis of which a key role is played by the natural and adaptive immune system [11]. One of the conclusive evidence is leukocytosis, a significant change in the leukocyte composition and immunologically different subpopulations of blood peripheral (BP) lymphocytes, and also the level of proinflammatory and regulatory cytokines and chemokines in BP of patients with prediabetes and T2D [41].
In this regard, the main purpose of this report was to study prospectively the leukocyte composition of BP, immunophenotype of lymphocytes (CD3+ T, CD4+ T, CD8+ T, CD20+ and CD56+ cells), the level of proinflammatory (IL-1β, TNF-α) and regulatory cytokine (CD10) in BP of newly diagnosed patients with T2D and signs of obesity before and after its treatment with metformin.
Materials and methods
Initial clinical and immunological examinations were performed in 29 newly diagnosed patients with T2D. Of these, a relatively homogeneous group of 11 patients of both sexes aged 38 to 60 years with a body mass index (BMI) of 28 to 37 kg/m2 (an average of 33.2 ± 1.3 kg/m2) was selected for further study according to the existing classification with “signs of obesity”. The selected group of patients with T2D, who were subsequently prescribed metformin, did not include patients who had myocardial infarction, stroke, as well as malignancies, acute and chronic respiratory diseases, renal failure, etc. Diagnosis of T2D was established in accordance with the recommendations of WHO and IDF experts. Metformin was prescribed at a dose of 2000 mg per day (the dose was gradually titra–ted for 1 week). Patients were examined before and in 3 months after taking the drug. The control group consisted of 12 practically healthy normoglycemic people of the same age as the group of patients.
The glucose content in the blood was determined by the glucose oxidase method. Impaired glucose tolerance was diagnosed by glucose level from 7.8 to 11.1 mmol/L in 120 minutes after glucose loading. The level of glyca–ted hemoglobin (HbA1c) was determined by calorimetric method with thiobarbituric acid.
The total number of leukocytes was counted using a hematological analyzer. Identification and number of different types of leukocytes (leukocyte formula) were carried out in BP smears stained by Pappenheim using phosphate or cacodylate buffer (pH 6.85) per 200 cells. In some cases, large granulosa-containing lymphocytes (LGL), which are considered to be the morphological homolog of natural killer cells (NK cells), were isolated into a separate group.
The lymphocyte content of different immunophenotypes was determined by flow cytometer using the FACStar plus laser cytofluorimeter “Becton Dickenson” (USA). Mononuclear cells were isolated from the heparinized BP by differential centrifugation in a density gradient of ficoll-isopak or ficoll-urotrast according to A. Boyum method. In some cases, they were further incubated in a CO2 incubator for 1 hour to eliminate monocytes. The surface antigens of lymphocytes-expressing CD3 (all T cells), CD4 (T-inducers/helpers), CD8 (T-suppressors/killers), CD20 (B-lymphocytes) and CD56 (NK cells) were labeled with monoclonal antibodies, which were conjugated with fluorescence isothiocyanate (FITZ) or phycoerythrin (PE) for visualization from Becton Dickenson (USA), Dako Cytomation (Denmark) or RE Kavetsky Institute of Experimental Pathology, Oncology and Radiobiology of National Academy of Sciences of Ukraine. 25.000 cells in each sample were analyzed by the
FACStar plus cytofluorimeter.
The number of different cytokines (IL-1β, TNF-α and IL-10) was determined by the ELISA immunoassay using StarFax 3200 spectrophotometer with vertical beam from Star company (USA) and reagent kit of Diaclone (USA) and Vector BEST (Russia).
Statistical processing of the obtained data was carried out by the method of variational statistics using the standard statistical calculation package for the Libre Office Calc program. The significance of the differences in mean values was determined by paired Student’s t-test when comparing the group before treatment with the group after metformin treatment and the Student’s test was used for unrelated groups when compared with healthy subjects. Values of p < 0.05 were considered significant.
Results
Leukocyte composition of blood. A small but significant (p < 0.05) increase in the total number of leukocytes was found in the hematological study of patients with T2D before the beginning of their treatment with metformin, as compared to the control group, which agrees with our previous data [32, 41], as well as the results of other authors, conducted on extensive clinical material as shown in Table 1. This leukocytosis is mainly due to a significant increase in the absolute number of segmented nuclei neutrophils (p < 0.05) and monocytes (p < 0.05). Moreover, a more significant increase in the number of monocytes in BP in some subjects (up to 15 %) is associated with the most elevated BMI.
As a result of metformin treatment, a significant decrease in the total number of leukocytes in BP (p < 0.05) was observed in the majority of patients with newly diagnosed T2D, due to the absolute number of segmented nuclei neutrophils (p < 0.05) and the number of monocytes (p < 0.05). At the same time, a decrease in monocytes was associated with a decrease in BMI, which suggests that this decrease may be due in part to the weight loss at patients.
Immunophenotype of lymphocytes. In the study of immunophenotype of lymphocytes in BP of patients with T2D before their treatment with metformin, a statistically significant increase in the absolute and relative number of CD4+ T cells was found, as can be seen from Table 2, in comparison with the control group, especially in individuals with elevated BMI that was close to literature data on this issue [3, 32].
As a result of metformin therapy, a significant decrease in the relative (p < 0.05) and absolute (p < 0.05) content of CD4+ T cells occurred in most of the exami–ned patients, approaching to values close to the control group (Table 2). The absolute number of CD3+ T cells also decreases significantly. The absolute number of natural killer cells (CD56+ lymphocytes) in many patients treated with metformin was also increased, which suggests an increase in natural immunity in such patients.
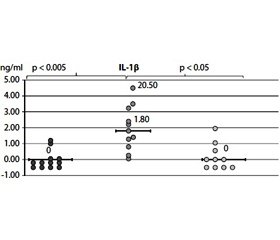
The content of cytokines. The content of IL-1β and TNF-α in healthy individuals and patients with newly diagnosed T2D is completely consistent with the results of our previous studies [40] and literature data [10, 11, 17]. A significant increase in the level of both IL-1β and TNF-α in BP was noted in the majority of patients with T2D compared with a group of healthy people, as can be seen from Fig. 1A and B. Thus, the content of IL-1β was increased in 8 of 11 patients (median — 1.8 pg/ml vs. 0 in the control, p < 0.05) and TNF-α in 7 of 11 patients (median 2.7 pg/ml vs. 0 in the control, p < 0.05). It is also important to note that in patients with the highest TNF-α content in the BP (6.5 pg/ml), had the highest BMI (37 kg/m2).
After metformin therapy, the majority of examined patients had a marked normalization of the content of both IL-1β and TNF-α in BP, the median content of IL-1β decreased from 1.8 pg/ml to 0 (p < 0.05 ), and TNF-α — from 2.7 pg/ml to 0 (p < 0.05) as can seen from Fig. 1. In addition, a decrease in TNF-α level was well correlated with a decrease in HbA1c.
Significant changes in content of the immunoregulatory cytokine IL-10, which is considered to have a potent immunosuppressive and anti-inflammatory effects, as can be seen from Fig. 1C, were not noted both in group of healthy individuals or in patients with T2D before and after their treatment with metformin. At the same time, it should be noted that 3 out of 10 treated patients had a higher level of IL-10 in the blood serum, two of whom simultaneously had the most pronounced decrease in BMI.
Discussion
It is well known that metformin is one of the effective antidiabetic drugs used in the treatment of T2D, but the mechanism of its favorable effect remains to be studi–ed little. This particularly applies to information about the role of the immune system in the mechanisms of the antidiabetic action of metformin in a sick person. At the same time, according to modern ideas, T2D refers to immunocompromised inflammatory diseases [11, 36]. The classical markers of inflammation, that is, leukocytosis due to neutrophilia and monocytosis, and a significant increase in pro-inflammatory cytokines (IL-1β, IL-6, IL-12, IL-17, IL-22, TNF-α, etc.) in BP [11, 30] are in favor of this hypothesis. There are also studies where has been established that an increase in the number of leukocytes and inflammatory cytokines in patients with IR and metabolic syndrome is a significant marker predicting the transition from prediabetes to clinically diagnosed T2D [13].
Our studies showed for the first time that after the course of treatment of patients with primary T2D, in many of them, together with the improvement of many clinical indices (decrease in the level of HbA1c, glucose, BMI, etc.), a significant normalization of a number of important markers of inflammation and immunity occurs. That is a decrease in the content of the total number of leukocytes, neutrophils and monocytes, the subpopu–lation of CD4+ T-lymphocytes, as well as a sharp decrease in the level of proinflammatory cytokines (IL-1β and especially TNF-α), indicating a pronounced anti-inflammatory property of metformin.
However, as is known, more than 80 % of patients with T2D simultaneously suffer from obesity, which is also considered as one of the types of nonspecific (low-grade) inflammation [14, 15, 34, 36] and is an integral cluster of IR and MC [35].
At the same time, in our examined patients with T2D BMI on the average was 33.2 ± 1.3 kg/m2, i.e. most of them had a “sign of obesity” and only two people had more than 35 kg/m2, i.e. there was “pronounced obesity”. At the same time, especially pronounced leukocytosis, neutrophilia and monocytosis were noted even in normoglycemic healthy people with obesity [2, 20, 23, 32, 33]. Consequently, the increase in the number of leukocytes due to neutrophils and monocytes, which was observed by us, suggests that these changes in the leukocyte composition in our examined patients with T2D are due not only to mechanisms specific for this disease, but also to the presence of excessive body weight. As a result of metformin therapy, both normalization of the leukocyte formula and weight body loss are noted. However, a complete association between these two processes was not revealed, which gives reason to think that the mechanism of favorable action of metformin is due to the effect of both factors.
A similar phenomenon was observed by us from the immunophenotype of lymphocytes, i.e. an increase in the CD4+ T cell count in BP of the examined patients with T2D, which is also observed in MS and IR, as well as in normoglycemic obese individuals [12, 36, 39]. And weight loss as a result of diet or surgical removal of FT excess, the content of this subpopulation of T-lymphocytes in BP decreases [27]. Recent immunological studies [7] have shown that this subpopulation of CD4+ T (Th17 and Th22 helper cells) consists of young and memorial CD4+ CD25+ FOXp3+ cells, whose number in obesity in visceral FT can be increased by almost 10 times [12, 36].
Normalization of CD4+ T cells content was revealed in our examined patients with T2D and excess body weight under metformin treatment, which also seems to be, to a certain extent, like the number of leukocytes, due to a decrease in the degree of obesity.
As a result of metformin therapy, the normalization of IL-1β and, especially, TNF-α in BP, which was associated with BMI decrease, was observed in the majority of the subjects.
It is also interesting to note that an increase in the level of TNF-α in circulation, positively correlates with the absolute amount of monocytes, both before and after metformin therapy, i.e. the higher the BMI, the higher the number of BP monocytes which agrees with the following data [36] that, with obesity, monocytes/macrophages migrate strongly to the inflammatory visceral FT, where secrete a large amount of TNF-α.
Consequently, a high content of IL-1β and TNF-α in BP of obese patients with T2D can be the result of synergism of overweight and specific processes characteristic only for the pathogenesis of T2D.
Evidence that the favorable therapeutic effect of metformin in T2D is largely due to its immuno-dependent and anti-inflammatory effects are also recent publications, showing that hypoglycemic effect and other widespread oral antidiabetic drugs (pioglitazone and rosiglitazone) are also mediated by their effect on immune mechanisms [4, 8, 18].
Conclusions
The obtained data suggest that the beneficial therapeutic effect of metformin in T2D is largely due to its effect on the functions of congenital and adaptive immunity, in which mechanism an essential role is played by obesity. At the same time, it is a good confirmation of the hypothe–sis that T2D should be attributed to diseases of inflammatory nature. Further deeper study of the role of the immune system in T2D is necessary to create new more effective drugs with anti-inflammatory and antidiabetic effects.
Conflicts of interests. Authors declare the absence of any conflicts of interests that might be construed to influence the results or interpretation of their manuscript.
Список литературы
1. Abbasi F., Chu J.W., McLaughlin T. et al. Effect of metformin treatment on multiple cardiovascular disease risk factors in patients with type 2 diabetes mellitus // Metabolism. — 2004. — Vol. 53, № 2. — P. 159-164.
2. Baig S., Rizi E.P., Shabeer M. et al. Acute meal challenge and modulation of postprandial immune-metabolic response in peripheral blood mononuclear cells, in lean, insulin-sensitive and obese, insulin-resistant Chinese // Diabetes. — 2015. — Vol. 64, Suppl. 1. — A467, 1805-P.
3. Bouter K.P., Meyling F.H., Hoekstra J.B. et al. Influence of blood glucose levels on peripheral lymphocytes in patients with diabetes mellitus // Diabetes Res. — 1992. — Vol. 19, № 2. — P. 77-80.
4. Brooks-Worrell B.M., Palmer J.P. Attenuation of islet-specific T cell responses is associated with C-peptide improvement in autoimmune type 2 diabetes patients // Clin. Exp. Immunol. — 2013. — Vol. 171, № 2. — P. 164-170.
5. Buse J.B., DeFronzo R.A., Rosenstock J. et al. The primary glucose-lowering effect of metformin resides in the gut, not the circulation: results from short-term pharmacokinetic and 12-week dose-ranging studies // Diabetes Care. — 2016. — Vol. 39, № 2. — P. 198-205.
6. Cameron A.R., Morrison V., McNeilly A.D. et al. The anti-inflammatory role of metformin // Diabetes. — 2015. — Vol. 64, Suppl. 1. — P. 1822 (A471).
7. Сhe T.T., Ren Y., Liu S.F. Expression of circulating CD4+CD25+ FOXP3+ regulatory T cells in obese patients // Diabetologia. — 2013. — Vol. 56, Suppl. 1. — A-563.
8. Coppola A., Caputo M.P., Pastore D. et al. Metformin inhibits leptin release induced by HMGB1 and exerts an anti-inflammatory action reducing TLR4/2 expression in T2D subjects // Diabetes. — 2015. — Vol. 64, Suppl. 1. — P. 1830 (A473).
9. De la Cuesta-Zuluaga J., Mueller N.T., Corrales-Agudelo V. et al. Metformin is associated with higher relative abundance of mucin-degrading akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut // Diabetes Care. — 2016. — Vol. 40, № 1. — P. 54-62.
10. Dinarello C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases // Blood. — 2011. — Vol. 117, № 14. — P. 3720-3732.
11. Donath M.Y. Multiple benefits of targeting inflammation in the treatment of type 2 diabetes // Diabetologia. — 2016. — Vol. 59, № 4. — P. 679-682.
12. Fabbrini E., Cella M., McCartney S.A. et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals // Gastroenterology. — 2013. — Vol. 145, № 2. — P. 366-374.
13. Ford E.S. Leukocyte count, erythrocyte sedimentation rate, and diabetes incidence in a national samples of US adults // Am. J. Epidemiol. — 2002. — Vol. 155, № 1. — P. 57-64.
14. Graham G.G., Punt J., Arora M. et al. Clinical pharmacokinetics of metformin // Clin. Pharmacokinet. — 2011. — Vol. 50, № 2. — P. 81-98.
15. Gregor M.F., Hotamisligil G.S. Inflammatory mechanisms in obesity // Annu. Rev. Immunol. — 2011. — Vol. 29. — P. 415-445.
16. Hattori Y., Hattori K., Hayashi T. Pleiotropic benefits
of metformin: macrophage targeting its anti-inflammatory mechanisms // Diabetes. — 2015. — Vol. 64, № 6. — P. 1907-1909.
17. Herder C., Peltonen M., Koenig W. et al. Anti-inflammatory effect of lifestyle changes in the Finnish Diabetes Prevention Study // Dialectologies. — 2009. — Vol. 52, № 3. — P. 433-442.
18. Hong S.J., Kim S.T., Kim T.J. et al. Cellular and molecular changes associated with inhibitory effect of pioglitazone on neointimal growth in patients with type 2 diabetes after zotarol–imus-eluting stent implantation // Arteriosclerosis, Thrombosis, and Vascular Biology. — 2010. — Vol. 30. — P. 2655-2665.
19. Inzucchi S.E., Bergenstal R.M., Buse J.B. et al. Mana–gement of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) // Diabetes Care. — 2012. — Vol. 35, № 6. — P. 1364-1379.
20. Kim J.A., Park H.S. White blood cell count and abdominal fat distribution in female obese adolescents // Metabolism. — 2008. — Vol. 57, № 10. — P. 1375-1379. 21. Konopka A.R., Esponda R.R., Robinson M.M. et al.
Hyperglucagonemia mitigates the effect of metformin on glucose production in prediabetes // Cell Rep. — 2016. — Vol. 15, № 7. — P. 1394-1400.
22. Kűhtreiber W.M., Burger D., Reinhold III P.E. et al. He–terogeneity in type 1 diabetics is defined by contrasting C-peptide declines, autoreactive T cell burdens, and metabolic differences // Diabetes. — 2015. — Vol. 64, № 4. — P. 1105-1107.
23. Kullo I.J., Hensrud D.D., Allison T.G. Comparison of numbers of circulating blood monocytes in men grouped by body mass index (< 25, 25 to < 30, > or = 30) // Am. J. Cardiol. — 2002. — Vol. 89, № 12. — P. 1441-1443.
24. Lamanna C., Monami M., Marchionni N., Mannucci E. Effect of metformin on cardiovascular events and mortality: a meta-analysis of randomized clinical trials // Diabetes Obes Metab. — 2011. — Vol. 13, № 3. — P. 221-228.
25. Luo T., Nocon A., Fry J. et al. AMPK activation by metformin suppresses abnormal extracellular matrix remodelling in adipose tissue and ameliorates insulin resistance in obesity // Diabetes. — 2016. — Vol. 65, № 8. — P. 2295-2310.
26. Mulherin A.J., Oh A.H., Kim H. et al. Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell // Endocrinology. — 2011. — Vol. 152, № 12. — P. 4610-4619.
27. O’Rourke R.W., White A.E., Metcalf M.D. et al. Hypo–xia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells // Diabetologia. — 2011. — Vol. 54, № 6. — P. 1480-1490.
28. Орленко В.Л., Зак К.П. Лечение аналогами глюкагоноподобного пептида-1 — прорыв в терапии сахарного диабета 2-го типа // Міжнародний ендокринологічний журнал. — 2014. — № 4(60). — С. 112-117.
29. Pernicova I., Korbonits M. Metformin — mode of action and clinical implications for diabetes and cancer // Nat. Rev. Endocrinol. — 2014. — Vol. 10, № 3. — P. 143-156.
30. Pham M.N., Hawa M.I., Pfleger C. et al. Pro- and anti-inflammatory cytokines in latent autoimmune diabetes in adults, type 1 and type 2 diabetes patients: Action LADA 4 // Diabetologia. — 2011. — Vol. 54. — P. 1630-1638.
31. Sang R. Mechanism of metformin: a tale of two sites // Diabetes Care. — 2016. — Vol. 39, № 2. — P. 187-189.
32. Саенко Я.А., Зак К.П., Попова В.В., Семионова Т.А. Лейкоцитарный состав и иммунофенотип лимфоцитов крови у женщин, больных сахарным диабетом 2-го типа, с ожирением // Міжнародний ендокринологічний журнал. — 2016. — № 5(77). — С. 13-19.
33. Schipper H.S., Nuboer R., Prop S. et al. Systemic inflammation in childhood obesity: circulating inflammatory mediators and activated CD14++ monocytes // Diabetologia. — 2012. — Vol. 55, № 10. — P. 2800-2810.
34. Seyhan A., Nunes-Lopez Yu., Garufi G. Differences in serum cytokine concentration in lean and obese individuals with prediabetes and type 2 diabetes // Diabetes. — 2015. — Vol. 64, Suppl. 1. — A472, 1825-P.
35. Simmons R.K., Alberti K.G.M.M., Gale E.A.M. et al. The metabolic syndrome: useful concept or clinical tool? Report of a WHO Expert Consultation // Diabetologia. — 2010. — Vol. 53. — P. 600-605.
36. Tsai S., Clemente-Casares X., Revelo X.S. et al. Are obesity-related insulin resistance and type 2 diabetes autoimmune diseases? // Diabetes. — 2015. — Vol. 64, № 6. — P. 1886-1897.
37. Tseng E., Yeh H.C., Maruthur N.M. Metformin use in prediabetes among U.S. adults, 2005–2012 // Diabetes Care. — 2017. — Vol. 40, № 7. — P. 887-893.
38. Wang Q., Zhang M., Torres G. et al. Metformin suppresses diabetes-accelerated atherosclerosis via the inhibition of Drp1-mediated mitochondrial fission // Diabetes. — 2017. — Vol. 66. — P. 193-205.
39. Womack J., Tien P.C., Feldman J. et al. Obesity and immune cell counts in women // Metabolism. — 2007. — Vol. 56, № 7. — P. 998-1004.
40. Зак К.П., Попова В.В. Предсказание развития сахарного диабета 1-го типа и диагностика его асимптомной фазы с помощью аутоантител к островкам Лангерганса поджелудочной железы у человека задолго до возникновения у него заболевания // Міжнародний ендокринологічний журнал. — 2016. — № 7(79). — С. 11-21.
41. Зак К.П. Роль нейтрофильных лейкоцитов в патогенезе сахарного диабета 1-го типа у человека (аналитический обзор с включением собственных данных) // Міжнародний ендокринологічний журнал. — 2016. — № 2(74). — P. 130-139.

/52-1.jpg )
/53-1.jpg )