Международный эндокринологический журнал Том 13, №8, 2017
Порівняльна характеристика активності фібринолізу в умовах експериментального перитоніту та його розвитку на тлі цукрового діабету
Авторы: F.V. Grynchuk, A.F. Grynchuk, V.V. Maksimiuk
Higher State Education Institution of Ukraine “Bukovinian State Medical University”, Chernivtsi, Ukraine
Рубрики: Эндокринология
Разделы: Справочник специалиста
Резюме
Актуальність зумовлена недостатнім вивченням стану фібринолітичних реакцій при поєднанні цукрового діабету (ЦД) з гострим перитонітом (ГП), що дедалі частіше трапляється в практиці. Мета дослідження: вивчення особливостей фібринолітичної активності при ГП, що розвивається на тлі ЦД. Матеріали та методи. 100 білих нелінійних щурів. ГП моделювали черезстравохідною перфорацією шлунка. ЦД моделювали уведенням 1,6% розчину алоксану. Вивчали сумарну фібринолітичну активність (СФА), неферментаційну (НФА) та ферментаційну (ФФА) фібринолітичну активність плазми крові. Тварини були поділені на групи: 1-ша — інтактні тварини з моделями ГП, 2-га — тварини з моделями ГП на тлі ЦД. Результати. Активність фібринолізу в тварин із моделями ЦД переважала таку в інтактних. Через 6 год з часу моделювання ГП фібринолітична активність зростала. У 1-й групі приріст був меншим. Через 12 год СФА, НФА і ФФА у 2-й групі різко збільшилися і значно переважали. У 1-й групі значуще збільшилася ФФА. Співвідношення НФА/ФФА знижувалось у обох групах. Через 24 год СФА у 1-й групі незначно зросла. У 2-й групі всі показники істотно збільшилися. Співвідношення НФА/ФФА у 1-й групі зростало, а у 2-й групі знижувалось. Через 48 год СФА і співвідношення НФА/ФФА у 1-й групі практично не змінились. У 2-й групі параметри СФА, НФА і ФФА статистично значуще переважали, а ФФА продовжувала зростати. Висновки. При експериментальному ЦД виявлено зростання активності фібринолізу з перевагою ферментаційних механізмів. При експериментальному ГП спостерігається активація фібринолізу зі збереженням рівноваги між його ланками впродовж 24 годин. Розвиток ГП у тварин з моделями ЦД вже через 6 годин суттєво відрізняється кількісними характеристиками фібринолітичної активності плазми крові, що проявляється її надмірним зростанням, розвитком дисбалансу між ланками фібринолізу, неконтрольованим наростанням ФФА з ознаками виникнення синдрому дисемінованого внутрішньосудинного згортання крові через 24 години. Підґрунтям виявлених відмінностей є зміни функціональної активності фібринолітичної системи, зумовлені впливом ЦД, що, окрім змін у системі гемостазу, створює передумови для порушень механізмів активації, міграції та взаємодії ефекторних клітин, процесів проліферації тощо.
Актуальность обусловлена недостаточным изучением состояния фибринолитических реакций при сочетании сахарного диабета (СД) с острым перитонитом (ОП), которое все чаще случается в практике. Цель исследования: изучение особенностей фибринолитической активности при ОП, развивающемся на фоне СД. Материалы и методы. 100 белых нелинейных крыс. ОП моделировали чрезпищеводной перфорацией желудка. СД моделировали введением 1,6% раствора аллоксана. Изучали суммарную фибринолитическую активность (СФА), неферментационную (НФА) и ферментационную (ФФА) фибринолитическую активность плазмы крови. Животные были разделены на группы: 1-я — интактные животные с моделями ОП, 2 — животные с моделями ОП на фоне СД. Результаты. Активность фибринолиза у животных с моделями СД превышала таковую у интактных. Через 6 ч с момента моделирования ОП фибринолитическая активность возрастала. В 1-й группе прирост был меньше. Через 12 ч СФА, НФА и ФФА во 2-й группе резко увеличились и значительно преобладали. В 1-й группе значимо увеличилась ФФА. Соотношение НФА/ФФА снижалось в обеих группах. Через 24 ч СФА в 1-й группе незначительно выросла. Во 2-й группе все показатели существенно увеличились. Соотношение НФА/ФФА в 1-й группе возрастало, а во 2-й группе — снижалось. Через 48 ч СФА и соотношение НФА/ФФА в 1-й группе практически не изменились. Во 2-й группе параметры СФА, НФА и ФФА статистически значимо преобладали, а ФФА продолжала расти. Выводы. При экспериментальном СД выявлено повышение активности фибринолиза с преобладанием ферментационных механизмов. При экспериментальном ОП наблюдается активация фибринолиза с сохранением равновесия между его звеньями в течение 24 часов. Развитие ОП у животных с моделями СД уже через 6 часов существенно отличается по количественным характеристикам фибринолитической активности плазмы крови, что проявляется ее чрезмерным увеличением, развитием дисбаланса между звеньями фибринолиза, неконтролируемым нарастанием ФФА с признаками возникновения синдрома диссеминированного внутрисосудистого свертывания крови через 24 часа. Основой выявленных различий являются изменения функциональной активности фибринолитической системы, обусловленные влиянием СД, что, кроме изменений в системе гемостаза, создает предпосылки для нарушений механизмов активации, миграции и взаимодействия эффекторных клеток, процессов пролиферации и др.
Background. Actuality is determined by understudied fibrinolytic reactions in case of diabetes mellitus (DM) with acute peritonitis (AP) which is to be found in practice more frequent. Objective of the study was to investigate the features of fibrinolytic activity in AP developed on the background of DM. Materials and methods. 100 albino outbred rats. AP was simulated through the esophageal perforation of the stomach. DM was modeled by the 1.6% alloxan solution injection. During the study, total (TFA), non-enzymatic (NFA) and enzymatic fibrinolytic activity (EFA) of the blood plasma was studied. The animals were divided into such groups: 1 — intact animals with AP models; 2 — animals with models of AP and underlying DM. Results. The activity of fibrinolysis in animals with DM models was higher than that of intact animals. Six hours after the AP have been induced, the fibrinolytic activity increased. There was a less augmentation in group 1. TFA, NFA and EFA in group 2 sharply increased and prevailed significantly in 12 hours. EFA significantly increased in group 1. NFA/EFA ratio was decreasing in both groups. TFA in group 1 slightly increased in 24 hours. All of the indicators in group 2 increased significantly. While the ratio of NFA/EFA in group 1 was increasing, in group 2 it was decreasing. TFA and NFA/EFA ratio in group 1 remained more or less constant in 48 hours. The parameters of TFA, NFA and EFA statistically significantly predominated in group 2, and EFA continued to grow. Conclusions. The increase in the fibrinolytic activity of the blood plasma with the fermentation mechanisms predomination have been found in experimental diabetes mellitus. The activation of fibrinolysis with balance maintenance between its links within 24 hours has been observed in case of experimental acute peritonitis. In 6 hours, the development of acute peritonitis in animals with simulated diabetes mellitus differs substantially in terms of its quantitative characteristics of the fibrinolytic activity of the blood plasma, which is shown by its excessive increase, development of imbalance between the links of fibrinolysis, uncontrolled increase in the activity of fermentation mechanisms with disseminated intravascular coagulation syndrome in 24 hours. The basis for the differences that have been detected are the changes in the functional activity of the fibrinolytic system caused by diabetes mellitus influence that, in addition to changes in the hemostasis system, provide the grounds for disorders of mechanisms of activation, migration and interaction of effector cells, processes of proliferation, etc.
Ключевые слова
цукровий діабет; перитоніт; коморбідність; фібринолітична система
сахарный диабет; перитонит; коморбидность; фибринолитическая система
diabetes mellitus; peritonitis; comorbidity; fibrinolytic system
Introduction
The incidence of diabetes mellitus (DM) is constantly growing all over the world in recent years [1, 2]. The number of patients with acute peritonitis associa–ted with DM is constantly growing [3] respectively. The mechanisms of development of such comorbid pathological state are still unrevealed. In addition, the chan–ges of fibrinolytic system (FS) have not been studied yet. The importance of such researches is stipulated by the role of FS components within the inflammation process development, peritonitis in particular [4–6]. The FS activity changes are an integral part of mechanisms of DM development at the same time [7, 8]. Therefore, the investigation of FS reactions within acute peritonitis developing against the ground of diabetes mellitus appears to be rather topical.
Objective of the study — to study the features of changes in the fibrinolytic activity of blood plasma wit–hin acute peritonitis developing against the ground of diabetes mellitus.
Materials and methods
The research has been carried out on 100 albino outbred rats, with the weight of 180 to 200 g. The animals were divided into 2 groups, each of the group consisted of 50 rats. The first group was formed by intact animals. The second one — animals with simulated DM. 40 animals of each group had medically induced peritonitis.
Peritonitis was simulated according to the common method through the esophageal perforation of the sto–mach with the help of a special device [9]. DM was simulated by subcutaneous introduction of 1.6% aloxane solution on distilled water in the dose of 16 mg per 100 kg of mass [10]. The main criterion of DM was the blood glucose presence within the range of 5.39 ± 0.25 mmol/l (in intact animals 3.21 ± 0.53 mmol/l, p < 0.01). Peritonitis was induced approximately 3 months after diabetes had been simulated. Before modeling peritonitis, as well as in 6, 12, 24, 48 hours from the moment of its inducement, blood was taken for analysis.
While carrying out the study the researchers kept to the basic guideline of Vancouver Convention (1979, 1994) concerning biomedical experiments. The animals were taken out of the experiment by decapitation. All manipulations were performed under the sevorane anesthesia. The Bioethics Committee of HSEE of Ukraine “Bukovinian State Medical University”, the Ministry of Public Health of Ukraine found the work to be done according to the basic moral and legal principle while conducting the clinical-experimental medi–cal research.
Total fibrinolytic activity (TFA), non-enzymatic (NFA) and enzymatic fibrinolytic activity (EFA) of blood plasma that determined by the level of azofibrin lysis by O.L. Kukharchuk method were studied [11].
The hypothesis of normal data distribution (Gaus–sian distribution) was tested in selections by Shapiro-Wilk criterion. Verification of the hypothesis of average data equality was carried out by Wilcoxon and Mann-Whitney-Wilcoxon criterion. The results of the study were statistically processed by the Microsoft® Office Excel (build 11.5612.5703) tables and programs for statistical calculations Statgraphics Plus 5.1 Enterprise edition (®Statistical Graphics corp. 2001).
Results
The activity of all fibrinolysis elements with simu–lated DM statistically significantly prevailed over those of the intact animals. To our mind, increase of plasma fibrinolytic activity could be compensatory in nature underlying hypercoagulation commonly found in DM [12].
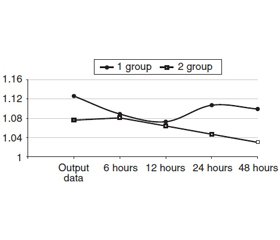
In 6 hours since peritonitis was modeled, FA star–ted increasing in both animal groups. However, all of TFA (fig. 1), EFA (fig. 1) and NFA in group 1 were increasing statistically significantly. Whereas group 2 was affected by a minor increase, this was probably due to the high baseline. FA was increasing in group 1, mainly at the expense of EFA (fig. 4). The interaction between different fibrinolysis bars in group 2 was mainly not changed.
In 12 hours FA of plasma was increasing. The parame–ters of all TFA indicators in group 2 increased statistically significantly and prevailed predominantly. There was a meaningful increase of EFA in group 1. The ratio between EFA and NFA decreased in both groups. Such dynamics is indicative of an increasing activity of the fibrinolytic system with the fermentation mechanisms predominance in response to peritonitis progression.
In 24 hours FA of plasma increased significantly. Whereas in group 2 the activity of fibrinolysis increased statistically significantly. At the same time, the ratio between EFA and NFA was increasing in group 1 showing the non-enzymatic mechanisms of fibrinolysis predominance. In group 2 the ratio decreased being indicative of the fermentation fibrinolysis activation.
In 48 hours FA plasma and the ratio of different fibrinolysis elements did not change. The parameters of EFA, NFA and TFA in group 2 statistically significantly prevailed and the activity of fermentation fibrinolysis continued to increase.
Discussion
FA decrease is considered to be the characteristic of DM [13, 14]. However, patients in clinics receive the treatment which is intended to correct the glucose level, in particular insulin, which causes FA suppression being associated with the activation of contrinsular mechanisms [15]. Taking into account a regulatory role of the fibrinolytic system in the implementation of protective function against inflammation [16], the identified FA of plasma increase in animals with DM models can be considered as one of the most important features changing peritonitis development. Such changes can occur through the influence of the fibrinolytic system factors on the proliferation mechanisms, which interrupt the processes of the inflamed place [6] delimitation as well as the influence on activation factors and cells migration — inflammation effectors [17, 18].
The increase of FA of plasma in case of peritonitis is a natural process, which is caused by different factors, among which are components of the complement and calicreatin-kinin system, immune complexes, growth of the activity of the coagulation system, etc. [5, 19, 20]. At the same time, in addition to hypercoagulation changes compensation, the important mechanisms of inflammation progress are associated with the fibrinolytic activation. Plasma activates growth factors, C8 — a complement fraction [1, 16]. Direct plasmin effect on the endothelium improves the cells migration, effectors of inflammation in the place [21], and the products of enzymatic degradation of fibrin are the activators of immunocompetent cells and chemoattractants and they can play the role of opsonins [16]. Thus, the lack of proper enzymatic fibrinolysis activation in animals with DM models in 6 hours after peritonitis inducement serves as a precondition to regulate disorders of the inflammatory process.
Further peritonitis progress is provided with a growing activation of the fibrinolytic system. The predominant growth of EFA in animals with DM models is indicative of high plasminogen activity and its activators and a significant level of plasmin in blood plasma [22]. The liver, bone marrow and kidneys [16] are known to be one of the most significant physiological sources of plasminogen. Their functions are affected in DM cases [1, 2], moreover, they are affected even more in peritonitis cases because of toxic damage [3, 6]. The study enables us to suggest, that a high level of EFA in group 2 is due to the initiation of other plasminogen donators, activated leukocytes, endothelial cells, microorganisms, broken tissues, etc. A high level of EFA in group 2 contribute to a significant number of circulating activators of plasminogen of different origin — blood, tissue, endothelial, bacterial, etc [23–25].
FA of plasma was increasing during 24 hours in group 1, mainly, due to the non-enzymatic factors. Taking into account a direct connection between NFA level and the amount of thrombin [26, 27], it may be interpreted as the consequence of coagulation system activation, which is aimed at restraining and delimiting the inflammatory process in the peritoneal cavity. It can explain a slight increase of EFA as well.
A significant increase of NFA in 24 hours in group 2 is indicative of hypercoagulative changes in blood [16, 27], whereas a significant increase in FFA level is indicative of the development of the initial stage of disseminated intravascular coagulation syndrome [28]. Considering the duration of peritonitis which causes disorders of the liver functions, being the main source of factors and inhibitors of EFA, such EFA increase also confirms the development of unlimited fibrinolysis, which has the nature of a cascade of autocatalytic progressive reactions.
The absence of significant changes in the indicators studied in group 1 in 48 hours is evidenced by the balance between the coagulation and anti-coagulation systems, on the one hand, and by the functional stabi–lity of factors — regulators of fibrinolysis, on the other. The superiority of EFA is being observed at the same time, which might be the first sign of imbalance of the fibrinolytic system. The reduction of NFA parameters in group 2, which is indicated by decrease of the thrombin content, is a sign of development of disseminated intravascular coagulation syndrome against the ground of FFA increase [28], occurrence of the syndrome disturbs the functioning of organs and tissues, liver and visceral peritoneum in particular, which represent antiproteina–se barrier [16, 22]. On this background, the plasmin of plasma, which is known to be a major factor of enzymatic fibrinolysis, easily goes into the tissue, in particular, into the peritoneum, which leads to disorders of proliferation processes, resulting in unrestrained spread of inflammation in the peritoneal cavity.
Conclusions
1. The increase in the fibrinolytic activity of blood plasma with the fermentation mechanisms predomination have been found in experimental diabetes mellitus case.
2. The activation of fibrinolysis with balance maintenance between its links within 24 hours has been observed in experimental acute peritonitis case.
3. In 6 hours, the development of acute peritonitis in animals with simulated diabetes mellitus differs substantially in its quantitative characteristics of the fibrinolytic activity of plasma blood, which is shown by its excessive increase, development of imbalance between the links of fibrinolysis, uncontrolled increase of the activity of fermentation mechanisms with disseminated intravascular coagulation syndrome in 24 hours.
4. The basis of the differences that have been detec–ted are the changes in the functional activity of the fibrinolytic system caused by diabetes mellitus influence that, in addition to changes in the hemostasis system, provide the grounds for disorders of mechanisms of activation, migration and interaction of effector cells, processes of proliferation, etc.
Conflicts of interests. Authors declare the absence of any conflicts of interests that might be construed to influence the results or interpretation of their manuscript.
Information on the contribution of each author:
Grynchuk F.V. The concept and design of the study.
Grynchuk A.F. The collection and processing of data, text writing.
Maksymyuk V.V. Analysis of the obtained data.
Список литературы
1. Сахарный диабет. Иммунитет. Цитокины / Зак К.П., Тронько М.Д., Попова В.В., Бутенко А.К. — К.: Книга-плюс, 2015. — 488 с.
2. Excess Mortality among Persons with Type 2 Diabetes / М. Tancredi, А. Rosengren, А.М. Svensson [et al.] // N. Engl. J. Med. — 2015. — Vol. 373, № 18. — Р. 1720-1732.
3. Гострий перитоніт на сучасному етапі — проблеми, здобутки і перспективи / І.Ю. Полянський, Ф.В. Гринчук, В.В. Білоокий [та ін.] // Клінічна анатомія і оперативна хірургія. — 2014. — Т. 13, № 1. — С. 83-87.
4. Comparison of recombinant human thrombomodulin and gabexate mesylate for treatment of disseminated intravascular coagulation (DIC) with sepsis following emergent gastrointe–stinal surgery: a retrospective study / T. Akahoshi, H. Sugimori, N. Kaku [et al.] // Eur. J. Trauma. Emerg. Surg. — 2015. — Vol. 41, № 5. — P. 531-538.
5. Effects of a TREM-like transcript 1-derived peptide du–ring hypodynamic septic shock in pigs / M. Derive, A. Boufenzer, Y. Bouazza [et al.] // Shock. — 2013. — Vol. 39, № 2. — P. 176-182.
6. Бочаров А.В. Тканинна фібринолітична активність в органах очеревинної порожнини при експериментальному жовчному перитоніті / А.В. Бочаров // Буковинський медичний вісник. — 2002. — № 1–2. — С. 46-49.
7. Wu R. Diverse coagulopathies in a rabbit model with diffe–rent abdominal injuries / R. Wu, L.G. Peng, H.M. Zhao // World. J. Emerg. Med. — 2017. — Vol. 8, № 2. — P. 141-147.
8. Behl T. Role of altered coagulation-fibrinolytic system in the pathophysiology of diabetic retinopathy / T. Behl, T. Velpandian, A. Kotwani // Vascul. Pharmacol. — 2017. — Vol. 92. — P. 1-5.
9. Пат. 4766 А Україна, МКИ А61В17/00, А61М27/00. Спосіб моделювання гострого перитоніту / Ф.В. Гринчук, І.Ю. Полянський; заявник і патентовласник Буковинський державний медичний університет. — № 2004031769; заявл. 11.03.04; опубл. 15.02.05, Бюл. № 2.
10. Shaw Dunn J. Experimental alloxan diabetes in the rat / J. Shaw Dunn, N.G.B. McLetchie // The Lancet. — 1943. — Vol. 242 (6265). — P. 384-387.
11. Кухарчук О.Л. Патогенетична роль та методи корекції інтегративних порушень гормонально-месенджерних систем регуляції гомеостазу натрію при патології нирок: автореф. дис. … д-ра мед. наук. — Одеса, 1996. — 36 с.
12. Aboonabi A. The effectiveness of antioxidant therapy in aspirin resistance, diabetes population for prevention of thrombosis / A. Aboonabi, I. Singh // Biomed. Pharmacother. — 2016. — Vol. 83. — P. 277-282.
13. Hypofibrinolysis in diabetes: a therapeutic target for the reduction of cardiovascular risk / K. Kearney, D. Tomlinson, K. Smith, R. Ajjan // Cardiovasc. Diabetol. — 2017. — Vol. 16, № 1. — P. 34. — Режим доступу до журн.: https://doi.org/10.1186/s12933-017-0515-9.
14. Is diabetes a hypercoagulable state? A critical appraisal / F. Pomero, M.N. Di Minno, L. Fenoglio, M. Gianni, W. Ageno, F. Dentali // Acta Diabetol. — 2015. — Vol. 52, № 6. — P. 1007-1016.
15. Грицюк А.И. Практическая гемостазиология / Грицюк А.И., Амосова Е.Н., Грицюк И.А. — К.: Здоров’я, 1994. — 256 с.
16. Монастирський В.А. Тромбін-плазмінова система — одна з основних регуляторних систем організму / Монастирський В.А.; Львів. нац. мед. ун-т ім. Д. Галицького, Наук. т-во ім. Т.Г. Шевченка. — Л.: Ліга-Прес, 2007. — 226 с.
17. Coagulation system changes associated with susceptibility to infection in trauma patients / E. Cole, R. Davenport, H. De’Ath, J. Manson, T. Brockamp, K. Brohi // J. Trauma. Acute. Care. Surg. — 2013. — Vol. 74, № 1. — P. 51-57.
18. Potential influences of complement factor H in autoimmune inflammatory and thrombotic disorders / J. Ferluga, L. Kouser, V. Murugaiah, R.B. Sim, U. Kishore // Mol. Immunol. — 2017. — Vol. 84. — P. 84-106.
19. Calibrated kallikrein generation in human plasma / D. Biltoft, J.J. Sidelmann, L.F. Olsen, Y. Palarasah, J. Gram // Clin. Biochem. — 2016. — Vol. 49, № 15. — P. 1188-1194.
20. Weiler H. Inflammation-associated activation of coagulation and immune regulation by the protein C pathway / H. Weiler // Thromb. Res. — 2014. — Vol. 133, Suppl. 1. — S. 32-34.
21. The Vascular Endothelium and Human Diseases / P. Rajendran, Th. Rengarajan, J. Thangavel [et al.] // Int. J. Biol. Sci. — 2013. — Vol. 9, № 10. — P. 1057-1069.
22. Effects of tissue plasminogen activator in experimentally induced peritonitis / B. Erginel, L. Oksuz, T. Erginel [et al.] // Ulus. Travma. Acil. Cerrahi. Derg. — 2014. — Vol. 20, № 1. — P. 7-11.
23. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice / B. McDonald, R.P. Davis, S.J. Kim [et al.] // Blood. — 2017. — Vol. 129, № 10. — P. 1357-1367.
24. Bhattacharya S. Bacterial plasminogen receptors utilize host plasminogen system for effective invasion and dissemination / S. Bhattacharya, V.A. Ploplis, F.J. Castellino // J. Мed. Biotechnol. — 2012. — 482096. — Режим доступу: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3477821/.
25. Gebbink M.F. Tissue-type plasminogen activator-media–ted plasminogen activation and contact activation, implications in and beyond haemostasis / M.F. Gebbink // J. Thromb. Haemost. — 2011. — Vol. 9, Suppl. 1. — S. 174-181.
26. Longstaff C. Basic mechanisms and regulation of fibrinolysis / C. Longstaff, K. Kolev // J. Thromb. Haemost. — 2015. — Vol.13, Suppl. 1. — S. 98-105.
27. Li X.The role of heparin in sepsis: much more than just an anticoagulant / X. Li, X. Ma // Br. J. Haematol. — 2017. — Vol. 179, № 3. — P. 389-398.
28. Venugopal A. Disseminated intravascular coagulation / A. Venugopal // Indian J. Anaesth. — 2014. — Vol. 58, № 5. — P. 603-608.

/140-1.jpg)
/141-1.jpg)
/141-2.jpg)