Резюме
У статті розглядаються питання впливу тіаміну та його похідних на перебіг ускладнень цукрового діабету (ЦД). Tіамін слугує коферментом для транскетолази, піруватдегідрогенази й комплексів α-кетоглутаратдегідрогенази, ферменти яких відіграють фундаментальну роль у внутрішньоклітинному метаболізмi глюкози. У літературі повідомляється про взаємозв’язки між тіаміном і ЦД. Рівні тіаміну й активність залежних від тіаміну ферментів знижені у хворих на ЦД. Генетичні дослідження дають можливість встановити зв’язуючі ланки між тіаміном і ЦД. Встановлено, що тіамін і його деривати запобігають активації біохімічних процесів (посилене виділення поліоловим шляхом, надмірне утворення кінцевих продуктів глікування, активація протеїнкінази C і посилення гексозамінового шляху біосинтезу), спричинених гіперглікемією за ЦД. Підкреслюється значення тіаміну при ендотеліальних судинних хворобах при ЦД (мікро- і макроангіопатія), порушеннях ліпідного обміну, при ретино-, нефро-, кардіо- і нейропатії.
В статье рассматриваются вопросы влияния тиамина и его производных на течение осложнений сахарного диабета (СД). Tиамин служит коферментом для транскетолазы, пируватдегидрогеназы и комплексов α-кетоглутаратдегидрогеназы, ферменты которых играют фундаментальную роль во внутриклеточном метаболизме глюкозы. В литературе сообщается о взаимосвязях между тиамином и СД. Уровни тиамина и активность зависимых от тиамина ферментов снижены у больных СД. Генетические исследования дают возможность установить связующие звенья между тиамином и СД. Установлено, что тиамин и его дериваты предотвращают активацию биохимических процессов (усиленное выделение полиоловым путем, избыточное образование конечных продуктов гликирования, активация протеинкиназы C и усиление гексозаминового пути биосинтеза), вызванных гипергликемией при СД. Подчеркивается значение тиамина при эндотелиальных сосудистых болезнях при СД (микро- и макроангиопатия), нарушениях липидного обмена, при ретино-, нефро-, кардио- и нейропатии.
Thiamіne acts as a coenzyme for transketolase and for the pyruvate dehydrogenase and α-ketoglutarate dehydrogenase complexes, whіch enzymes play a fundamental role in intracellular glucose metabolіsm. The relatіonship between thiamіne and diabetes mellitus (DM) has been reported in the literature. Thiamine levels and thiamine-dependent enzyme activities are reduced in DM. Genetic studies provide opportunity tо determine the relatiоnship between thiamine and DM. Thiamine and its derivatives were demonstrated to prevent the activation of the biоchemical pathways (increased flux through the polyol pathway, formation of advanced glycation end-products, activation of protein kinase C, and increased flux through the hexоsamine biosynthesis pathway) induced by hyperglycemia in DM. Thiamine definitively plays a rоle in the diabetic endothelial vascular diseases (micro- and macroangiopathy), lipid profile, retinоpathy, nephrоpathy, cardiоpathy, and neurоpathy.
Thiamine was the first of the water-soluble vitamins to be described. In 1884, Kanehiro Takaki (1849–1920), a surgeon general in the Japanese navy, rejected the previous germ theory for beriberi and hypothesized that the disease was due to insufficiencies in the diet. Switching diet on a navy ship, he discovered that substituting a diet of white rice only, with one also containing barley, meat, milk, bread, and vegetables nearly eliminated beriberi on a 9-month sea voyage. Not until 1905, after the anti-beriberi factor had been discovered in rice bran (removed by polishing into white rice) and in brown barley rice, Takaki’s experiment was rewarded. The specific connection to grain was made in 1897 by Christiaan Eijkman (1858–1930), a military doctor in the Dutch Indies, discovered that fowl fed on a diet of cooked, polished rice developed paralysis, which could be reversed by discontinuing rice polishing. Eijkman was eventually awarded the Nobel Prize in Physiology and Medicine in 1929, because his observations led to the discovery of vitamins. These compounds were named by Polish biochemist Casimir Funk. In 1911, Casimir Funk isolated the antineuritic substance from rice bran that he called a “vitamin” (on account of its containing an amino group). Dutch chemists, Barend Coenraad Petrus Jansen (1884–1962) and his closest collaborator Willem Frederik Donath (1889–1957), went on to isolate and crystallize the active agent in 1926, which structure was determined by Robert Runnels Williams (1886–1965), a US chemist, in 1934. Thiamine (“sulfur-containing vitamin”) was synthesized in 1936 by the same group. Lohman and Schuster in 1937 showed that the diphosphorylated thiamine derivative (thiamine diphosphate, ThDP) was a cofactor required for the oxidative decarboxylation of pyruvate (a reaction now known to be catalyzed by pyruvate dehydrogenase), thereby elucidating the mechanism of action of thiamine in the cellular metabolism.
It is on the World Health Organization’s List of Essential Medicines, the most effective and safe medicines needed in a health system.
Thiamine is a colorless organosulfur compound with a chemical formula C12H17N4OS. Its structure consists of an aminopyrimidine and a thiazole ring linked by a methylene bridge. The thiazole is substituted with me–thyl and hydroxyethyl side chains. Thiamine is soluble in water, methanol, and glycerol and practically insoluble in less polar organic solvents. It is stable at acidic pH, but is unstable in alkaline solutions. Thiamine is unstable to heat, but stable during frozen storage. It is unstable when exposed to ultraviolet light.
Thiamine is released by the action of phosphatase and pyrophosphatase in the upper small intestine. At low concentrations, the process is carrier-mediated. At higher concentrations, absorption also occurs via passive diffusion. Active transport is greatest in the jejunum and ileum, but it can be inhibited by alcohol consumption or by folate deficiency. Decline in thiamine absorption occurs when intaking above 5 mg/day. On the serosal side of the intestine, vitamin discharge by those cells is dependent on Na+-dependent ATPase. The majority of thiamine in serum is bound to proteins, mainly albumin. Approximately 90 % of total thiamine in blood is in erythrocytes. A specific binding protein called –thiamine-binding protein has been identified in rat serum and is believed to be a hormone-regulated carrier protein important for tissue distribution of thiamine. Uptake of thiamine by cells of the blood and other tissues occurs via active transport and passive diffusion. About 80 % of intracellular thiamine is phosphorylated and most is bound to proteins. In some tissues, thiamine uptake and secretion appears to be mediated by a soluble thiamine transporter that is dependent on Na+ and a transcellular proton gradient.
Thiamine (vitamin B1) is an indispensable molecule for all known organisms. Its phosphate derivatives are involved in many cellular processes. The best-characterized form is thiamine pyrophosphate (TPP), a coenzyme in the catabolism of sugars and amino acids. In yeast, TPP is also required in the first step of alcoholic fermentation. All organisms use thiamine, but it is made only in bacteria, fungi, and plants. Animals must obtain it from their diet, and thus, for humans, it is an essential nutrient.
Apart from free thiamine five other phosphorylated and adenylated derivatives of thiamine are known: thiamine monophosphate (ThMP), thiamine diphosphate, also sometimes called thiamine pyrophosphate, thiamine triphosphate (ThTP), adenosine thiamine triphosphate, adenosine thiamine diphosphate.
The coenzyme role of ThDP is well-known and extensively characterized. The non-coenzyme action of thiamine and its derivatives has recently been identified to a number of proteins, which do not use the catalytic action of thiamine diphosphate. Malate dehydrogenase, glutamate dehydrogenase and pyridoxal kinase were identified as abundant proteins binding to thiamine or thiazolium-modified sorbents. Thiamine is not only a coenzyme for acetyl-CoA production, but also an allosteric regulator of acetyl-CoA metabolism including regulatory acetylation of proteins and acetylcholine biosynthesis.
No physiological role is known for thiamine monophosphate. Free cytosolic ThDP is generally hydrolyzed into ThMP, which is recycled to thiamine. No specific enzymes have been identified for the latter reactions and there is no known role for ThMP. Intracellular ThMP levels are generally much lower than ThDP levels. Ho–wever, ThMP seems to be excreted, probably by a process involving the reduced folate carrier.
In mammalian cells, the synthesis of ThDP, also known as thiamine pyrophosphate or cocarboxylase, is catalyzed by an enzyme called thiamine diphosphokinase. ThDP is the coenzyme for five key metabolic enzymes — mitochondrial pyruvate dehydrogenase complexes, oxoglutarate dehydrogenase complexes, 2 branched-chain acid dehydrogenase complexes, 2-hydroxyacyl-CoA –lyase 1 as well as the cytosolic trans–ketolase.
ThTP is found in nearly all organisms and is the only known triphosphorylated compound that is not a nucleotide. With two phosphoanhydride bonds, it is an energy-rich compound and as such it has been shown to be able to phosphorylate proteins. ThTP seems to be constitutively synthesized in animal cells, in E.coli it accumulates only in the absence of amino acids and therefore could be a signaling molecule involved in the adaptation to amino acid starvation. Though it was long thought that ThTP is synthesized by an ATP:ThDP phosphotransferase, the existence of such a mechanism has never been unambiguously demonstrated.
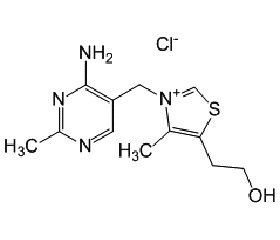
Benfotiamine (synonym S-benzoylthiamine O-monophosphate) is a so-called allithiamine, a member of the class of lipophilic thiamine derivatives, first identified in heated garlic in 1950. It was later confirmed that similar compounds could be formed using other Allium vegetables from compounds similar to allicin. The molecular formula of benfotiamine is C19H23N4O6PS.
The unique properties of the allithiamines result from the opening of thiamine thiazole ring upon reac–tion with sulphur compounds. Upon benfotiamine dephosphorylation in the intestinal tract a lipophilic molecule is produced, which readily diffuses across cell membranes and is absorbed much better than water soluble thiamine salts. This property allows for greater absorption both in the intestines and in target tissues as compared with thiamine itself. Benfotiamine is absorbed via passive diffusion through the intestinal mucosa and is rapidly converted to biologically active thiamine. Peak plasma concentrations of thiamine after oral benfotiamine administration are at least five times greater than those observed after oral administration of water-soluble thiamine salts. Half-life of benfotiamine is similar to thiamine salts, but bioavailabi–lity of benfotiamine eight days after administration is roughly 25 % of the original dose, about 3.6 times greater than after an oral dose of a thiamine salt. The increase in relative bioavailability is most significant in muscle (5-fold greater incorporation) and brain (25-fold increase), but thiamine from benfotiamine is also 10–40 % better incorporated in other organs, such as liver and kidney.
The relationship between thiamine and dіabetes mellitus (DM) has been reported in the literature. Significant proportion of healthy subjects (36–47 %) was reported as a thiamine-deficient in a hyperglycemic state. Low plasma thiamine level was noted in type 1 diabetic patients. In children, acute thiamine deficiency can be manifested by diabetic ketoacidosis, lactic acidosis and hyperglycemia [1]. Plasma thiamine level has been shown to be decreased by 76 % in type 1 and 75 % in type 2 diabetic patients. It was associated with increased renal clearance and fractional excretion of thiamine [2]. Furthermore, thiamine transporter protein concentration has been shown to be increased in erythrocyte membranes of type 1 and type 2 diabetic patients.
Dysfunction of endothelial cells has been known to play a major role in both micro- and macrovascular complications of DM. Thiamine reversed hyperglycemia-induced dysfunction in cultured endothelial cells [3]. Thiamine and benfotiamine have been demonstrated in vitro to counteract the da–maging effects of hyperglycemia on cultured vascular cells [4]. In addition, thiamine has been reported to improve endothelium vasodilation in patients with hyperglycemia [5]. Daily intake of thiamine positively correlated with the circulating level of endothelial progenitor cells and vascular endothelial function in type 2 diabetic patients [6].
Diabetic cardiomyopathy can progress toward overt heart failure with increased mortality. Benfotiamine improved functional recovery of the іnfarcted heart with prolonged survival and reduced cardiomyocyte apoptosis in diabetic mice [7]. High dose of thiamine rescues cardiomyocyte contractile dysfunction and it also prevented diastolic dysfunction, heart failure and cardiac fibrosis in diabetes-induced mіce models [8].
Nephropathy is a common complication of diabetes. It is characterized by the development of proteinuria and end-stage renal disease. In a double-blind placebo-controlled study, urinary albumin excretion was decreased in type 2 diabetic patients with microalbuminuria after receiving high-dose of thiamine for 3 months [9]. In another study with high dose of thiamine, the level of urіnary albumin decreased by 34 % in the type 2 diabetic patients [10].
Benfotiamine has shown to effect in the diabetic neuropathy patients with reduction in pain score and improve neurophysiological parameters [4]. Benfothiamine significantly reduced inflammatory (10–300 mg/kg) and neuropathic (75–300 mg/kg) nociception in non-diabetic and diabetic rats [7]. In a double blіnd, placebo-controlled, phase-III clinical study with benfotiamine in diabetic polyneuropathy, the improvement of neuropathy symptom score was more pronounced at the higher benfotiamine dose (600 vs. 300 mg) and increased with treatment duration [6].
Thiamіne and benfotiamine were reported to regulate the intracellular glucose and polyol pathway in bovine retinal pericytes cultured in high glucose [1]. Early and selective loss of pericytes and thickenіng of the basement membrane are hallmarks of diabetic retinopathy. Thiamine and benfotiamine prevent apoptosis induced by high glucose-conditioned extracellular matrix in human and bovіne retinal pericytes.
Thiamine definitively plays a role in the dіabetic endothelial vascular diseases (micro- and macroangiopathy), lipid profile, retinopathy, nephropathy, cardiopathy, and neuropathy.
Conflicts of interests. Authors declare no conflicts of interests that might be construed to influence the results or interpretation of their manuscript.
Список литературы
1. Luong K., Nguyen L.T.H. The impact of thiamine treatment in the diabetes mellitus // J. Clin. Med. Res. — 2012. — 4 (3). — P. 153-160.
2. Hammes H.P., Du X., Edelstein D., Taguchi T., Matsumura T., Ju Q., Lin J. et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy // Nat. Med. — 2003. — 9 (3). — P. 294-299.
3. Berrone E., Beltramo E., Solimine C., Ape A.U., Porta M. Regulation of intracellular glucose and polyol pathway by thiamine and benfotiamine in vascular cells cultured in high glucose // J. Biol. Chem. — 2006. — 281 (14). — P. 9307-9313.
4. Karachalias N., Babaei-Jadidi R., Rabbani N., Thornal–ley P.J. Increased protein damage in renal glomeruli, retina, nerve, plasma and urine and its prevention by thiamine and benfotiamine therapy in a rat model of diabetes // Diabetologia. — 2010. — 53 (7). — P. 1506-1516.
5. Babaei-Jadidi R., Karachalias N., Ahmed N., Battah S., Thornalley P.J. Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine // Diabetes. — 2003. — 52 (8). — P. 2110-2120.
6. Kohda Y., Shirakawa H., Yamane K., Otsuka K., Kono T., Terasaki F., Tanaka T. Prevention of incipient diabetic cardiomyopathy by high-dose thiamine // J. Toxicol. Sci. — 2008. — 33 (4). — P. 459-472.
7. Gonzalez-Ortiz M., Martinez-Abundis E., Robles-Cervantes J.A., Ramirez-Ramirez V., Ramos-Zavala M.G. Effect of thiamine administration on metabolic profile, cytokines and inflammatory markers in drug-naive patients with type 2 diabetes // Eur. J. Nutr. — 2011. — 50 (2). — P. 145-149.
8. Pácal L., Kuricová K., Kaňková K. Evidence for altered thiamine metabolism in diabetes: Is there a potential to oppose gluco- and lipotoxicity by rational supplementation? // World J. Diabetes. — 2014. — 5 (3). — P. 288-295; doi: 10.4239/wjd.v5.i3.288
9. Page G.L., Laight D., Cummings M.H. Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease // Int. J. Clin. Pract. — 2011. — 65. — P. 684-690.
10. Rabbani N., Thornalley P.J. Emerging role of thiamine therapy for prevention and treatment of early-stage diabetic nephropathy // Diabetes Obes. Metab. — 2011. — 13. — P. 577-583.