Международный эндокринологический журнал Том 16, №4, 2020
Вернуться к номеру
Correlation of vitamin D level with thyroid status and TSH antibody titers in patients with Graves’ disease
Авторы: V.I. Pankiv(1), T.Yu. Yuzvenko(1), S.M. Koval(2), K. Singh(1), I.V. Pankiv(3), Tarun Sehgal(4), O.M. Lytvinova(5)
(1) — Ukrainian Research and Practical Centre of Endocrine Surgery, Transplantation of Endocrine Organs
and Tissues of the Ministry of Health of Ukraine, Kyiv, Ukraine
(2) — L.T. Mala National Institute of Therapy of the NAMN Ukraine, State Institution, Kharkiv, Ukraine
(3) — Bukovinian State Medical University, Chernivtsi, Ukraine
(4) — Dr B.R. Ambedkar National Institute of Technology, Jalandhar, Punjab, India
(5) — National University of Pharmacy, Kharkiv, Ukraine
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
Актуальність. У багатьох дослідженнях встановлено зв’язок дефіциту вітаміну D з автоімунними захворюваннями. Доведено, що рівень вітаміну D у пацієнтів з автоімунними захворюваннями щитоподібної залози, включаючи хворобу Грейвса, був нижчим, ніж у пацієнтів із неавтоімунними тиреоїдними захворюваннями, наприклад токсичним вузловим зобом. Водночас в окремих статтях повідомляють про відсутність такої залежності між рівнем вітаміну D та автоімунними захворюваннями щитоподібної залози. Мета дослідження: порівняти рівень вітаміну D у сироватці крові в пацієнтів із хворобою Грейвса та осіб контрольної групи, оцінити співвідношення вмісту вітаміну D із функціональним станом щитоподібної залози і титром антитіл до рецептора тиреотропного гормону (ТТГ). Матеріали та методи. Під спостереженням перебували 48 пацієнтів із хворобою Грейвса та 24 здорові особи контрольної групи. Усім обстеженим проводили гормональні дослідження, які містили рівень ТТГ сироватки, тироксину, вільного трийодтироніну, паратиреоїдного гормону, а також визначали рівень іонізованого кальцію, 25-гідроксивітаміну D (25(OH)D), титру антитіл до рецептора ТТГ. Результати. У пацієнтів із хворобою Грейвса спостерігався вірогідно нижчий рівень 25(OH)D (16,3 ± 1,4 нг/мл) порівняно з показниками контрольної групи (22,8 ± 1,6 нг/мл) (p = 0,024). Рівні ТТГ і титри антитіл до рецептора ТТГ вірогідно відрізнялися між групою осіб із хворобою Грейвса з дефіцитом вітаміну D (25(OH)D < 20 нг/мл) та групою хворих із хворобою Грейвса без дефіциту вітаміну D (25(OH)D ≥ 20 нг/мл). Обсяг щитоподібної залози істотно не відрізнявся між цими групами хворих. Рівень вітаміну D у сироватці крові вірогідно взаємопов’язаний із вмістом ТТГ і титром антитіл до рецептора ТТГ у пацієнтів із хворобою Грейвса. Висновки. Рівень вітаміну D у сироватці крові вірогідно нижчий у пацієнтів із хворобою Грейвса. У цих пацієнтів встановлено вірогідну кореляцію між вмістом вітаміну D і рівнями ТТГ й антитіл до рецептора ТТГ.
Актуальность. Во многих исследованиях установлена связь дефицита витамина D с аутоиммунными заболеваниями. Доказано, что уровень витамина D у пациентов с аутоиммунными заболеваниями щитовидной железы, включая болезнь Грейвса, был ниже, чем у пациентов с неаутоиммунными тиреоидными заболеваниями, например токсическим узловым зобом. В то же время в отдельных статьях сообщают об отсутствии такой зависимости между уровнем витамина D и аутоиммунными заболеваниями щитовидной железы. Цель исследования: сравнить уровень витамина D в сыворотке крови у пациентов с болезнью Грейвса и лиц контрольной группы, оценить соотношение содержания витамина D с функциональным состоянием щитовидной железы и титром антител к рецептору тиреотропного гормона (ТТГ). Материалы и методы. Под наблюдением находились 48 пациентов с болезнью Грейвса и 24 здоровых лица контрольной группы. Всем обследованным проводили гормональные исследования, содержащие уровень ТТГ сыворотки, свободного тироксина, свободного трийодтиронина, паратиреоидного гормона, а также определяли уровень ионизированного кальция, 25-гидроксивитамина D (25(OH)D), титр антител к рецептору ТТГ. Результаты. У пациентов с болезнью Грейвса наблюдался достоверно более низкий уровень 25(OH)D (16,3 ± 1,4 нг/мл) по сравнению с показателями контрольной группы (22,8 ± 1,6 нг/мл) (p = 0,024). Уровни ТТГ и титры антител к рецептору ТТГ достоверно отличались между группой лиц с болезнью Грейвса с дефицитом витамина D (25(OH)D < 20 нг/мл) и группой больных с болезнью Грейвса без дефицита витамина D (25(OH)D ≥ 20 нг/мл). Объем щитовидной железы существенно не отличался между этими группами больных. Уровень витамина D в сыворотке крови достоверно взаимосвязан с концентрацией ТТГ и титром антител к рецептору ТТГ у пациентов с болезнью Грейвса. Выводы. Уровень витамина D в сыворотке крови достоверно ниже у пациентов с болезнью Грейвса при сравнении с показателями контрольной группы. У этих пациентов установлена достоверная корреляция между содержанием витамина D и уровнями ТТГ и антител к рецептору ТТГ.
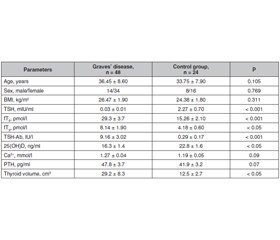
Background. Various studies have shown the association of vitamin D deficiency with autoimmune diseases. Studies have found that vitamin D levels in patients with autoimmune thyroid diseases including Graves’ disease were lower than that in patients with non-autoimmune thyroid diseases such as toxic nodular goiter. Some studies have reported no such relationship between vitamin D level and autoimmune thyroid diseases. The purpose of the study: to compare serum vitamin D level in patients with Graves’ disease versus age and sex matched controls, to assess the correlation of vitamin D with thyroid status and thyrotropin receptor antibody titers. Materials and methods. 48 patients with Graves’ disease and 24 age and sex matched healthy individuals were recruited. Hormonal investigations that included serum thyroid stimulating hormone (TSH), free thyroxine (fT4), free triiodothyronine (fT3), as well as calcium, 25-hydroxyvitamin D (25(OH)D), parathyroid hormone (PTH), thyroid stimulating hormone receptor antibody (TSH-Ab) were done for all subjects. Results. The patients with Graves’ disease had significantly lower 25(OH)D levels (16.3 ± 1.4 ng/ml) as compared to control subjects (22.8 ± 1.6 ng/ml) (p = 0.024). TSH levels and TSH-Ab titers differed significantly between vitamin D deficient Graves’ disease group (25(OH)D < 20 ng/ml) and vitamin D non deficient Graves’ disease group (25(OH)D ≥ 20 ng/ml). Thyroid volume did not differ significantly between these groups. Serum vitamin D level correlated significantly with TSH and TSH-Ab titers in patients with Graves’ disease. Conclusions. Serum vitamin D levels are significantly lower in patients with Graves’ disease. Significant correlation between vitamin D and TSH and TSH-Ab titers was found in these patients.
хвороба Грейвса; вітамін D; антитіла до рецептора ТТГ
болезнь Грейвса; витамин D; антитела к рецептору ТТГ
Graves’ disease; vitamin D; TSH-Ab titers
Introduction
Materials and methods
Results
Discussion
Conclusions
- Villa A., Corsello A., Cintoni M. et al. Effect of vitamin D supplementation on TSH levels in euthyroid subjects with autoimmune thyroiditis. Endocrine. 2020;10.1007/s12020-020-02274-9. doi: 10.1007/s12020-020-02274-9.
- Krysiak R., Kowalcze K., Okopień B. The effect of vitamin D on thyroid autoimmunity in euthyroid.men with autoimmune thyroiditis and testosterone deficiency. Pharmacol. Rep. 2019. 71(5). Р. 798-803. doi: 10.1016/j.pharep.2019.04.010.
- Yasuda T., Okamoto Y., Hamada N. et al. Serum vitamin D levels are decreased in patients without remission of Graves’ disease. Endocrine. 2013. 43(1). Р. 230-232. doi: 10.1007/s12020-012-9789-6.
- Ma J., Wu D., Li C. et al. Lower Serum 25-Hydroxyvitamin D Level is Associated With 3 Types of Autoimmune Thyroid Diseases. Medicine (Baltimore). 2015. 94(39). e1639. doi: 10.1097/MD.0000000000001639.
- Zhang H., Liang L., Xie Z. Low vitamin D status is associated with increased thyrotropin-receptor antibody titer in Graves’ disease. Endocr. Pract. 2015. 21. Р. 258-263. doi: 10.4158/EP14191.OR.
- Kmieć P., Sworczak K. Vitamin D in thyroid disorders. Exp. Clin. Endocrinol. Diabetes. 2015. 123(7). Р. 386-393. doi: 10.1055/s-0035-1554714.
- Kim D. The Role of Vitamin D in Thyroid Diseases. Int. J. Mol. Sci. 2017. 18(9). Р. 1949. doi: 10.3390/ijms18091949.
- Misharin A., Hewison M., Chen C.R., Lagishetty V., Aliesky H.A., Mizutori Y. et al. Vitamin D deficiency modulates Graves’ hyperthyroidism induced in BALB/c mice by thyrotropin receptor immunization. Endocrinology. 2009. 150. Р. 1051-1060. doi: 10.1210/en.2008-1191.
- Planck T., Shahida B., Malm J., Manjer J. Vitamin D in Graves’ disease: Levels, correlation with laboratory and clinical parameters, and genetics. Eur. Thyroid J. 2018. 7(1). Р. 27-33. doi: 10.1159/000484521.
- Yamashita H., Noguchi S., Takatsu K., Koike E., Murakami T., Watanabe S. et al. High prevalence of vitamin D deficiency in Japanese female patients with Graves’ disease. Endocr. J. 2001. 48. Р. 63-69. doi: 10.1507/endocrj.48.63.
- Xu M.Y., Cao B., Yin J., Wang D.F., Chen K.L., Lu Q.B. Vitamin D and Graves’ disease: a meta-analysis update. Nutrients. 2015. 7(5). Р. 3813-3827. doi: 10.3390/nu7053813.
- Pankiv V.I., Yuzvenko T.Yu., Pankiv I.V. Type 2 diabetes mellitus and subclinical hypothyroidism: focusing on the role of cholecalciferol. Problems of Endocrine Pathology. 2019. 2. Р. 46-51. doi: 10.21856/j-PEP.2019.2.07.
- Li X., Wang G., Lu Z., Chen M., Tan J., Fang X. Serum 25-hydroxyvitamin D predict prognosis in radioiodine therapy of Graves’ disease. J. Endocrinol. Invest. 2015. 38. Р. 753-759. doi: 10.1007/s40618-015-0252-4.
- Mangaraj S., Choudhury A.K., Swain B.M., Sarangi P.K., Mohanty B.K., Baliarsinha A.K. Evaluation of Vitamin D Status and its Impact on Thyroid Related Parameters in New Onset Graves’ Disease- A Cross-sectional Observational Study. Indian J Endocrinol Metab. 2019. 23(1). Р. 35-39. doi: 10.4103/ijem.IJEM_183_18.
- Ahn H.Y., Chung Y.J., Cho B.Y. Serum 25-hydroxyvitamin D might be an independent prognostic factor for Graves’ disease recurrence. Medicine (Baltimore). 2017. 96(31). e7700. doi: 10.1097/MD.0000000000007700.

/17.jpg)
/18.jpg)