Международный эндокринологический журнал Том 17, №2, 2021
Вернуться к номеру
Епідеміологія автоімунного тиреоїдиту
Авторы: Кравченко В.І.(1), Товкай О.А.(2), Раков О.В.(1), Тронько М.Д.(1)
(1) — ДУ «Інститут ендокринології та обміну речовин ім. В.П. Комісаренка НАМН України», м. Київ, Україна
(2) — Український науково-практичний центр ендокринної хірургії, трансплантації ендокринних органів і тканин МОЗ України, м. Київ, Україна
Рубрики: Эндокринология
Разделы: Справочник специалиста
Версия для печати
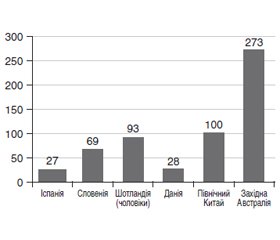
Наведений огляд літератури з питання епідеміології автоімунного тиреоїдиту (АІТ). В огляді розглянуті етіологічні чинники автоімунного ураження щитоподібної залози (ЩЗ). У виникненні ушкодження клітин ЩЗ важливе значення надається утворенню антитіл та лімфоїдній інфільтрації залози. Відзначено, що генетичні фактори передують виникненню патологічних змін. Втрата імунної толерантності до автоантигенів ЩЗ — тиреоїдної пероксидази (TПO), тиреоглобуліну (TГ) є основою для розвитку АІТ. Зазначена важлива роль оксидативного стресу і реактивних форм кисню в патогенезі захворювання. Показано, що на своєму початку АІТ перебігає безсимптомно, утворення антитіл до ТПО і ТГ передує виникненню захворювання і може свідчити про латентний АІТ. Поширеність латентного АІТ відрізняється в різних країнах світу і сягає від 2 до 20 %, причому серед жінок вона була в 4–6 разів вища, ніж у чоловіків. Згодом латентний АІТ переходить у субклінічний та явний тиреоїдит із гіпотиреозом. Захворюваність на маніфестний АІТ у різних країнах становить від 27 до 273 на 100 000 населення. Нерідко захворювання розпочиналося в дитячому та підлітковому віці. Частота патології, включаючи латентний субклінічний та маніфестний АІТ, у цієї когорти населення, за даними різних авторів, сягає від 0,3 до 9,6 %. Вагітність також супроводжується наявністю антитіл до ТПО, але зі зменшеною агресією клітинних елементів й антитіл до ЩЗ. Післяпологовий період характеризується загостренням захворювання. В Україні захворюваність населення на АІТ становить 43,1 на 100 000, поширеність майже в 10 разів вища — 421,2 на 100 000. Зроблено висновок, що АІТ є найбільш поширеним органоспецифічним автоімунним захворюванням.
A review of the literature on the epidemiology of autoimmune thyroiditis (AT) is presented. This review examines the etiological factors of autoimmune thyroid damage. In case of damage to thyroid cells, the formation of antibodies and lymphoid infiltration of the gland is of great importance. It is noted that genetic factors precede the occurrence of pathological changes. Loss of immune tolerance to thyroid autoantigens such as thyroid peroxidase (TPO), thyroglobulin underlies the development of AT. The role of oxidative stress and reactive oxygen species is important in the pathogenesis of the disease. It is shown that at the beginning, AT is asymptomatic and the formation of TPO and thyroglobulin antibodies precedes the onset of the disease and may indicate latent AT. The prevalence of latent AT varies from country to country and ranges from 2 to 20 %, and among women it was 4–6 times higher than among men. Subsequently, latent AT progresses to subclinical and overt thyroiditis with hypothyroidism. The incidence of manifest AT in various countries is from 27 to 273 per 100,000 population. Often, the disease began in childhood and adolescence. The frequency of pathology, including latent subclinical and manifest AT, in this cohort of the population according to different authors is from 0.3 to 9.6 %. Pregnancy was also accompanied by the presence of TPO antibodies but with reduced aggression of cellular elements and antibodies to the thyroid gland. The postpartum period was characterized by exacerbation of the disease. In Ukraine, the incidence of AT is 43.1 per 100,000, the prevalence is almost 10 times higher — 421.2 per 100,000. It is concluded that AT is the most common organ-specific autoimmune disease.
щитоподібна залоза; автоімунний тиреоїдит; антитиреоїдні антитіла; оксидативний стрес; епідеміологія; післяпологовий тиреоїдит; огляд
thyroid gland; autoimmune thyroiditis; antithyroid antibodies; oxidative stress; epidemiology; postpartum thyroiditis; review
/63_2.jpg)
/65.jpg)
- Unal E., Akın A., Yıldırım R., Demir V., Yildiz I., Kenan Haspolat Y. Association of subclinical hypothyroidism with dyslipidemia and increased carotid intima-media thickness in children. J. Clin. Res. Pediatr. Endocrinol. 2017. 9. 144-149. doi: 10.4274 jcrpe.3719.
- Vukovic R., Zeljkovic A., Bufan B., Spasojevic-Kalimanovska V., Milenkovic T., Vekic E. Hashimoto Thyroiditis and Dyslipidemia in Childhood: A Review Front. Endocrinol. (Lausanne). 2019. 10. P. 868. doi: 10.3389fendo.2019.00868.
- Głowinska-Olszewska B., Borysewicz-Sańczyk H., Sawicka B., Klonowska B., Charemska D., Żelazowska-Rutkowska B., Bossowski A. Does Hashimoto’s Thyroiditis Increase the Risk of Cardiovascular Disease in Young Type 1 Diabetic Patients? Front Endocrinol (Lausanne). 2020. 11. P. 431. doi: 10.3389fendo.2020.00431.
- Jeppesen R., Eriksen Benros M. Autoimmune Diseases and Psychotic Disorders Front Psychiatry. 2019. 10. P. 131. doi: 10.3389 fpsyt.2019.00131.
- Sliwinska A., Fumuso P., Stringer B., Ansar M., Baldwin J. Hashimoto Encephalopathy With Status Epilepticus Monitoring. Cureus. 2020 Dec. 12(12). P. e11857. doi: 10.7759 cureus.11857.
- Hashimoto H. Zur Kenntnis der lymphomatösen Veränderung der Schilddrüse (Struma lymphomatosa). Archiv für klinische Chirurgie, Berlin 97. 1912. P. 219-248.
- Zakharchenko T.F., Kravchenko V.I. Peculiarities of innate and adaptive immunity in the pathogenesis of thyroid autoimmune diseases (part 1). Mìžnarodnij endokrinologìčnij žurnal. 2020. 16. 7. doi: org/10.22141 2224-0721.16.7.2020. 219011. (in Ukrainian)
- Hwangbo Y., Park Y.J. Genome-Wide Association Studies of Autoimmune Thyroid Diseases, Thyroid Function, and Thyroid Cancer. Endocrinol. Metab. 2018. 33. 175-184. https:// doi.org/10.3803/ EnM. 2018.33.2.175.
- Panicker V. Genetics of thyroid function and disease. Clin. Biochem. Rev. 2011. 32. 165-175.
- The Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007. 447. 661-678. https://doi.org/10.1038/nature05911.
- Simmonds M.J., Gough S.C. The search for the genetic contribution to autoimmune thyroid disease: the never ending story? Brief. Funct. Genomics. 2011. 10. 77-90. doi: 10.1093/bfgp/elq036.
- Brown R.S. Autoimmune thyroid disease: unlocking a complex puzzle. Curr. Opin. Pediatr. 2009. 21. 523-528. doi: 10.1097/MOP.0b013e32832cf824.
- Jacobson E.M., Tomer Y. The genetic basis of thyroid autoimmunity. Thyroid. 2007. 17. 949-961. doi: 10.1089/thy.2007.0153.
- Teft W.A., Kirchhof M.G., Madrenas J. A molecular perspective of CTLA-4 function. Annual. Review of Immunology. 2006. 24. 65-97. doi: 10.1146/annurev.immunol.24.021605.090535.
- Ajjan R.A., Kemp E.H., Waterman E.A., Watson P.F., Endo T., Onaya T., Weetman A.P. Detection of binding and blocking autoantibodies to the human sodium-iodide symporter in patients with autoimmune thyroid disease. J. Clin. Endocrinol. Metab. 2000. 85. 2020-2027. doi: org/10.1210/jcem.85.5.6526.
- Yoshida A., Hisatome I., Taniguchi S., Shirayoshi Y., Yamamoto Y., Miake J., Ohkura T. et al. Pendrin is a novel autoantigen recognized by patients with autoimmune thyroid diseases. J. Clin. Endocrinol. Metab. 2009. 94. 442-448. doi: 10.1210/jc.2008-1732.
- Caturegli P., De Remigis A., Rose N.R. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun. Rev. 2014. 13(4–5). 391-397. doi: 10.1016/j.autrev.2014.01.007.
- Weetman A.P. The immunopathogenesis of chronic autoimmune thyroiditis one century after Hashimoto. Eur. Thyroid J. 2013. 1. 243-250. doi: 10.1159/000343834.
- Dong Y.H., Fu D.G. Autoimmune thyroid disease: mechanism, genetics and current knowledge. Eur. Rev. Med. Pharmacol. Sci. 2014. 18. 3611-3618. PMID: 25535130.
- Cogni G., Chiovato L. An overview of the pathogenesis of thyroid autoimmunity. Hormones. 2013. 12. 19-29. doi: 10.1007/BF03401283.
- Brent G.A. Environmental exposures and autoimmune thyroid disease. Thyroid. 2010. 20. 755-761. doi: 10.1089/thy.2010.1636.
- Eschler D.C., Hasham A., Tomer Y. Cutting edge: the etiology of autoimmune thyroid diseases. Clin. Rev. Allergy Immunol. 2011. 41. 190-197. doi: 10.1007/s12016-010-8245-8.
- Belin R.M., Astor B.C., Powe N.R., Ladenson P.W. Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration in the third National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 2004. 89. 6077-6086. 567. doi: 10.1210/jc.2004-0431.
- Carle A., Bulow Pedersen I., Knudsen N., Perrild H., Ovesen L., Banke Rasmussen L. et al. Smoking cessation is followed by a sharp but transient rise in the incidence of overt autoimmune hypothyroidism a population-based, case-control study. Clin. Endocrinol. (Oxf.) 2012. 77. 764-772. 571. doi: 10.1111/j.1365-2265.2012.04455.x.
- Effraimidis G., Strieder T.G., Tijssen J.G., Wiersinga W.M. Natural history of the transition from euthyroidism to overt autoimmune hypo- or hyperthyroidism: a prospective study. Eur. J. Endocrinol. 2011. 164. 107-113. 574. doi: 10.1530/EJE-10-0785.
- Effraimidis G., Tijssen J.G., Wiersinga W.M. Discontinuation of smoking increases the risk for developing thyroid peroxidase antibodies and/or thyroglobulin antibodies: a prospective study. J. Clin. Endocrinol. Metab. 2009. 94. 1324-1328. doi: 10.1210/jc.2008-1548.
- Caturegli P., Ruggere C. Karl Hurthle! Now, who was he? Thyroid. 2005. 15. 121-123. doi: 10.1089/thy.2005.15.121.
- Shawky M., Sakr M. Hurthle Cell Lesion: Controversies, Challenges, and Debates. Indian J. Surg. 2016. 78(1). 41-48. doi: 10.1007/s12262-015-1381-x.
- Kure S., Ohashi R. Thyroid Hürthle Cell Carcinoma: Clinical, Pathological, and Molecular Features Cancers (Basel). 2021. 26. doi: 10.3390/cancers13010026.
- Thieblemont N., Wright H.L., Edwards S.W., Witko-Sarsat V. Human neutrophils in autoimmunity. Semin. Immunol. 2016. 28(2). 159-173. doi: 10.1016/j.smim.2016.03.004.
- Pankiv V.I., Yuzvenko T.Yu., Pankiv I.V. Type 2 diabetes mellitus and subclinical hypothyroidism: focusing on the role of cholecalciferol. Problems of Endocrine Pathology. 2019. 2. 46-51. doi: 10.21856/j-PEP.2019.2.07.
- Rodríguez Y., Rojas M., Monsalve D.M. et al. Latent autoimmune thyroid disease. J. Transl. Autoimmun. 2020. 3. doi: 10.1016 j.jtauto.2020.100038.
- Tomer Y., Menconi F. Type 1 diabetes and autoimmune thyroiditis: the genetic connection. Thyroid. 2009. 19. 2. 99-102. doi: 10.1089 thy.2008.1565.
- Hwang G.B., Yoon J.S., Park K.J., Lee H.S., Hwang J.S. Prevalence of autoimmune thyroiditis in patients with type 1 diabetes: a long-term follow-up study. Ann. Pediatr. Endocrinol. Metab. 2018. 23, 1. 33-37. doi: 10.6065apem.2018. 23.1.33.
- Staii A., Mirocha S., Todorova-Koteva K., Glinberg S., Jaume J.C. Hashimoto thyroiditis is more frequent than expected when diagnosed by cytology which uncovers a pre-clinical state. Thyroid Res. 2010. 3. 11. doi: 10.1186 1756-6614-3-11.
- Caturegli P., De Remigis A., Rose N.R. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmunity Reviews. 2014. 13. 391-397. doi: 10.1016/j.autrev.2014.01.007.
- Thomsen H., Li X., Sundquist K., Sundquist J., Försti A., Hemminki K. Familial risks between Graves’ disease and Hashimoto thyroiditis and other autoimmune diseases in the population of Sweden. J. Transl. Autoimmun. 2020. Jun 1. doi: 10.1016 j.jtauto.2020.100058.
- Ragusa F., Fallahi P., Elia G. et al. Hashimotos’ Thyroiditis: epidemiology, pathogenesis, clinic and therapy. Journal Pre-proof PII: S1521-690X (19)30118-6 doi: org 10.1016j.beem. 2019.101367.
- McLeod D.S., Cooper D.S. The incidence and prevalence of thyroid autoimmunity. Rew. Cooper Endocrine. 2012. 42. 252-265. doi: 10.1007 s12020-012-9703-2.
- Teng W., Shan Z., Teng X. et al. Effect of iodine intake on thyroid diseases in China. N. Engl. J. Med. 2006. 354. 26. 2783-2793. doi: 10.1056/NEJMoa054022.
- Sun X., Shan Z., Teng W. Effects of Increased Iodine Intake on Thyroid Disorders. Endocrinol. Metab. (Seoul). 2014. 29(3). 240-247. doi: 10.3803EnM.2014.29.3.240.
- Burek C.L., Talor M. Environmental Triggers of Autoimmune Thyroiditis. J. Autoimmun. 2009. 33(3–4). 183-189. doi: 10.1016 j.jaut.2009.09.001.
- Dragin N., Bismuth J., Cizeron-Clairac G. et al. Estrogen-mediated downregulation of AIRE influences sexual dimorphism in autoimmune diseases. J. Clin. Invest. 2016. 126. 1525-1537. doi: 10.1172 JCI81894.
- Thompson E., Nicodemus-Johnson J., Kim K. et al. C21 OMICS IN LUNG DISEASE: puberty-associated and methylation changes in females are near estrogen responsive genes and implicated in immune processes. Am. J. Respir. Crit. Care Med. 2017. 195. A4970.
- Bliddal S., Henrik Nielsen C., Feldt-Rasmussen U. Recent advances in understanding autoimmune thyroid disease: the tallest tree in the forest of polyautoimmunity. F1000Res. 2017. 6. 1776. doi: 10.12688f1000research.11535.1.
- Eaton W., Pedersen M.G., Atladóttir H.O., Gregory P., Rose N., Mortensen P.B. The prevalence of 30 ICD-10 autoimmune diseases in Denmark. Immunol. Res. 2010. 47. 13. 228-231. doi: 10.1007 s12026-009-8153-2.
- Eaton W., Rose N.R., Kalaydjian A., Pedersen M.G., Mortensen P.B. Epidemiology of autoimmune diseases in Denmark. J. Autoimmun. 2007. 29. 1. 1-9. doi: 10.1016 j.jaut.2007.05.002.
- Kaloumenou I., Mastorakos G., Alevizaki M. et al. Thyroid autoimmunity in schoolchildren in an area with long-standing iodine sufficiency: correlation with gender, pubertal stage, and maternal thyroid autoimmunity. Thyroid. 2008. 18. 747-754. doi: 10.1089/thy.2007.0370.
- Mariotti S., Prinzis A., Ghiani M. et al. Puberty is associated with a marked increase of the female sex predominance in chronic autoimmune thyroiditis. Horm. Res. 2009. 72. 52-56. doi: 10.1159/000224341.
- Kyritsi E.M., Kanaka-Gantenbein C. Autoimmune Thyroid Disease in Specific Genetic Syndromes in Childhood and Adolescence. Front. Endocrinol. (Lausanne). 2020. 11. 543. doi: 10.3389/fendo.2020.00543.
- Kiyaev A.V., Savelyev L.I., Gerasimova L.Yu., Koroleva N.P., Boyarsky S.N., Tsvirenko S.V. Prevalence of thyroid diseases in children and adolescents in the iodine-deficient region. Clinical and experimental thyroidology. 2007. Т. 3. № 2. 33-38. doi: org/10.14341/ket20073233-38. (in Russian)
- Wasniewska M., Corrias A., Salerno M. et al. Thyroid function patterns at Hashimoto's thyroiditis presentation in childhood and adolescence are mainly conditioned by patients’ age. Horm. Res. Paediatr. 2012. 78. 232-236. doi: 10.1159/000343815.
- Aversa T., Corrias A., Salerno M. et al. Five-year prospective evaluation of thyroid function test evolution in children with Hashimoto’s thyroiditis presenting with either euthyroidism or subclinical hypothyroidism. Thyroid. 2016. 26. 1450-1456. doi: 10.1089/thy.2016.0080.
- Negro R., Formoso G., Mangieri T., Pezzarossa A., Dazzi D. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J. Clin. Endocrinol. Metab. 2006. 91(7). 2587-2591. doi: 10.1210/jc.2005-1603.
- Linding A.S., Jørn O. Early Pregnancy Thyroid Function Test Abnormalities in Biobank Sera from Women Clinically Diagnosed with Thyroid Dysfunction Before or After Pregnancy. Thyroid. 2017. 27(3). 451-459. doi: 10.1089/thy.2016.0542.
- Tingi E., Syed A., Kyriacou A., Mastorakos G. Benign thyroid disease in pregnancy: A state of the art review. Journal of Clinical & Translational Endocrinology. 2016. 6. 37-49. doi: 10.1016/j.jcte.2016.11.001.
- Okosieme O.E., Marx H., Lazarus J.H. Medical mana–gement of thyroid dysfunction in pregnancy and the postpartum. Expert Opin. Pharmacother. 2008. 9. 2281-2293. doi: 10.1517/14656566.9.13.2281.
- Stagnaro-Green A., Roman S.H., Cobin R.H., El-Harazy E., Alvarez-Marfany M., Davies T.F. Detection of at-risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. The Journal of the American Medical Association. 1990. 264(11). 1422-1425. PMID: 2118190.
- Feldt-Rasmussen U., Hoier-Madsen M., Rasmussen N.G., Hegedus L., Hornnes P. Anti-thyroid peroxidase antibodies during pregnancy and postpartum. Relation to postpartum thyroiditis. Autoimmunity. 1990. 6(3). 211-214. doi: 10.3109/08916939009041041.
- Negro R., Mestman J.H. Thyroid disease in pregnancy. Best Practice & Research Clinical Endocrinology & Metabolism. 2011. 25. 927-943. doi: 10.1016/j.beem.2011.07.010.
- Yang H., Shao M., Chen L. et al. Screening Strategies for Thyroid Disorders in the First and Second Trimester of Pregnancy in China 2014. PLoS One. 2014. 9(6). e99611. doi: 10.1371/PLoS One.2014.9(6).e99611.
- Almomin A., Mansour A. Spectrum of Thyroid Abnormalities among Pregnant Women in Basrah. PLoS One. 2014. 9(6). e99611. Jun 12. doi: 10.1371/journal.pone.0099611.
- Shahbazian H.B., Sarvghadi F., Azizi F. Clinical study Prevalence and characteristics of postpartum thyroid dysfunction in Tehran European Journal of Endocrinology. 2001. 145(4). 397-401. doi: 10.1530/eje.0.1450397.
- Stagnaro-Green A. Approach to the patient with postpartum thyroiditis. J. Clin. Endocrinol. Metab. 2012. 97. 334-342. dоi: 10.1210/jc.2011-2576.
- Walfish P.G., Meyerson J., Provias J.P., Vargas M.T., Papsin F.R. Prevalence and characteristics of post-partum thyroid dysfunction: results of a survey from Toronto, Canada. J. Endocrinol. Invest. 1992. 15. 265-272. doi: 10.1007/BF03348726.
- Hym F., Kologlu M., Collison K., Hall R., McGregor A.M. Postpartum thyroid dysfunction in Mid Glamorgan. Br. Med. J. (Clin. Res. Ed.). 1988. 23. 296(6617). 241-244. doi: 10.1136/bmj.296.6617.241.
- Payenok O.S., Vdovychenko Yu.P., Pankiv V.I. et al. Thyroid pathology and pregnancy. Lviv, 2020. 264 p. (in Ukrainian). ISBN 978-617-7336-62-3.
- Kaminskyi O.V., Pankiv V.I., Pankiv I.V., Afanasyev D.E. Vitamin D content in population of radiologically contaminated areas in Chernivtsi oblast (pilot project). Problems of radiation medicine and radiobiology. 2018. 23. 442-451. doi: 10.33145/2304_8336_2018_23_442_451.
- Kravchenko V.I., Andrusyshyna I.M., Luzanchuk I.A. Association Between Thyroid Hormone Status and Trace Elements in Serum of Patients with Nodular Goiter. Biol. Trace Elem. Res. 2020. 196. 2. 393-399. doi: 10.1007 s12011-019-01943-9.
- Rostami R., Nourooz-Zadeh S., Mohammadi A., Khalkhali H.R., Ferns G., Zadeh G.N. Serum Selenium Status and Its Interrelationship with Serum Biomarkers of Thyroid Function and Antioxidant Defense in Hashimoto’s Thyroiditis Antioxidants. Basel. 2020. 9(11). 1070. doi: 10.3390 antiox9111070.
- Mehl S., Sun Q., Görlich C.L. et al. Cross-sectional analysis of trace element status in thyroid disease. J. Trace Elem. Med. Biol. 2020. 196. 2. 393-399. doi: 10.1007 s12011-019-01943-9. PMID 31835129.
- Luzanchuk A., Kravchenko V.I., Polumbryk M.O., Tarachenko Y.M. Thyroid status, major and trace elements content in patients with autoimmune thyroiditis living in Chernobyl-affected areas of Zhytomyr region. Problems of Endocrine Pathology. 2020. 3. 54-62. doi: 10.21856/j-PEP.2020.3.07.
- Zimmermann M.B., Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015. 13. doi: 10.1016/S2213-8587(14)70225-6.
- Erdal M., Sahin M., Hasimi A., Uckaya G., Kutlu M., Saglam S. Trace Element Levels in Hashimoto Thyroiditis Patients with Subclinical Hypothyroidism. Biol. Trace Elem. Res. 2008. 123. 1-7. doi: 10.1007/s12011-008-8117-8.
- Rasic-Milutinovic Z., Jovanovic D., Bogdanovic G., Trifunovic J., Mutic J. Potential Influence of Selenium, Copper, Zinc and Cadmium on L-Thyroxine Substitution in Patients with Hashimoto Thyroiditis and Hypothyroidism. Exp. Clin. Endocrinol. Diabetes. 2017. 125(2). 79-85. doi: 10.1055/s-0042-116070.
- Feske S., Wulff H., Skolnik E.Y. Ion Channels in Innate and Adaptive Immunity. Annu. Rev. Immunol. Author manuscript; available in PMC 2016. Annu. Rev. Immunol. 2015. 33. 291-353. doi: 10.1146/annurev-immunol-032414-112212.
- Wang K., Wei H., Zhang W., Li Z. et al. Severely low serum magnesium is associated with increased risks of positive anti-thyroglobulin antibody and hypothyroidism: A cross-sectional study. Sci. Rep. 2018. 8. 9904. doi: 10.1038/s41598-018-28362-5.

/63.jpg)
/65_2.jpg)