Международный эндокринологический журнал Том 18, №2, 2022
Вернуться к номеру
Вірогідне зниження співвідношення вмісту нейтрофілів і лімфоцитів на тлі лікування інгібіторами натрійзалежного котранспортера глюкози 2-го типу у пацієнтів з цукровим діабетом 2-го типу
Авторы: Ozge Kurtkulagi
Abant Izzet Baysal University Hospital, Department of Internal Medicine, Bolu, Turkey
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
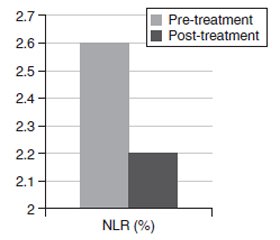
Актуальність. Інгібітори натрійзалежного котранспортера глюкози 2-го типу (іНЗКТГ-2) — нові терапевтичні засоби, доступні для лікування цукрового діабету 2-го типу (ЦД2). Ця група протидіабетичних засобів асоціюється зі зниженням глікованого гемоглобіну (HbA1c), глікемії натще, маси тіла та індексу маси тіла (ІМТ) у пацієнтів з ЦД2. Усі ці сприятливі ефекти також можуть бути пов’язані зі зменшенням запалення. Метою дослідження є порівняння отриманого з гемограми співвідношення нейтрофілів і лімфоцитів, нового маркера запалення, до та через 6 місяців після лікування іНЗКТГ-2 у пацієнтів з ЦД2. Крім того, завданням дослідження було порівняти рівень глікемії натще, глікованого гемоглобіну та інші метаболічні параметри, а також антропометричні показники (маса тіла, ІМТ) до та через 6 місяців після початку терапії іНЗКТГ-2. Матеріали та методи. Під спостереженням перебували пацієнти з ЦД2 у клініці внутрішніх хвороб університетської лікарні Abant Izzet Baysal у період з січня по грудень 2021 року. Порівнювали співвідношення нейтрофілів і лімфоцитів до та після лікування та інші параметри. Також були отримані лабораторні дані до та після лікування, включаючи вміст сечовини, креатиніну, глікемію натще, HbA1c, швидкість клубочкової фільтрації, аспартат- і аланінтрансамінази, натрій і калій плазми. Результати. Рівень глікемії натще знизився з 195 ± 72 мг/дл у період до лікування до 146 ± 53 мг/дл у період після лікування (p < 0,001). Відзначалося вірогідне зниження рівня HbA1c: з 9,1 ± 1,7 % у період до лікування до 7,7 ± 1,7 % у період після лікування (p < 0,001). Співвідношення нейтрофілів і лімфоцитів до лікування становило 2,6 ± 1,2 % і вірогідно знижувалося до 2,2 ± 0,6 % на шостому місяці терапії іНЗКТГ-2. Співвідношення нейтрофілів і лімфоцитів вірогідно зменшилося після лікування (p = 0,003). Висновки. Автор припускає, що співвідношення рівнів нейтрофілів і лімфоцитів може бути маркером зниження запального навантаження у пацієнтів з ЦД2, які отримують лікування іНЗКТГ-2.
Background. Sodium glucose cotransporter-2 inhibitors (SGLT2i) are novel therapeutic agents that became available in the treatment of type 2 diabetes mellitus (T2DM). This group of antidiabetic agents are associated with reduced glycated hemoglobin (HbA1c), fasting glucose, body weight and body mass index (BMI) in diabetic patients. All those beneficial effects may also be associated with a reduction in inflammatory burden. The purpose of the study is to compare neutrophil to lymphocyte ratio (NLR), a novel inflammatory marker derived from hemogram, before and 6 months after SGLT2i treatment in diabetic subjects. We also aimed to compare fasting glucose, HbA1c and other metabolic parameters as well as anthropometric measures (weight, BMI) before and 6 month after initiation of SGLT2i therapy. Materials and methods. The subjects with type T2DM that show up in internal medicine outpatient clinics of Abant Izzet Baysal University Hospital between January 2021 and December 2021 were enrolled to the study. Pretreatment and posttreatment NLR and other parameters were compared. We also obtained pretreatment and posttreatment laboratory data including urea, creatinine, fasting glucose, HbA1c, glomerular filtration rate, aspartate and alanine transaminases, plasma sodium and potassium. Results. Fasting glucose was reduced from 195 ± 72 mg/dl in pretreatment period to 146 ± 53 mg/dl in posttreatment period (p < 0.001). HbA1c was reduced from 9.1 ± 1.7 % in pretreatment period to 7.7 ± 1.7 % in posttreatment period (p < 0.001). The NLR before treatment was 2.6 ± 1.2 % before SGLT2i treatment and was reduced to 2.2 ± 0.6 % in 6th month of SGLT2i therapy. NLR was significantly decreased after treatment (p = 0.003). Conclusions. We suggest that NLR levels could be a marker of reduced inflammatory burden in T2DM subjects receiving SGLT2i treatment.
цукровий діабет 2-го типу; запалення; співвідношення нейтрофілів і лімфоцитів; інгібітори натрійзалежного котранспортера глюкози 2-го типу; глікемія натще; глікований гемоглобін
type 2 diabetes mellitus; inflammation; neutrophil to lymphocyte ratio; sodium glucose cotransporter-2 inhibitor; fasting glucose; HbA1c
Introduction
Materials and methods
Results
Discussion
Conclusions
- Cakir L., Aktas G., Enginyurt O. et al. Mean platelet volume increases in type 2 diabetes mellitus independent of HbA1c level. Acta Medica Mediterranea. 2014. 30. 425-428.
- Vernekar P.V., Vaidya K.A. Comparison of mean platelet volume in type 2 diabetics on insulin therapy and on oral hypoglycaemic agents. J. Clin. Diagn. Res. 2013. 7(12). 2839-40. doi: 10.7860/JCDR/2013/7636.3771.
- Kodiatte T.A., Manikyam U.K., Rao S.B., Jagadish T.M., Reddy M., Lingaiah H.K., Lakshmaiah V. Mean platelet volume in Type 2 diabetes mellitus. J. Lab. Physicians. 2012. 4(1). 5-9. doi: 10.4103/0974-2727.98662.
- Atak B., Aktas G., Duman T.T., Erkus E., Kocak M.Z., Savli H. Diabetes control could through platelet-to-lymphocyte ratio in hemograms. Rev. Assoc. Med. Bras. 2019. 65(1). 38-42. doi: 10.1590/1806-9282.65.1.38.
- Gasparyan A.Y., Ayvazyan L., Mukanova U., Yessirkepov M., Kitas G.D. The Platelet-to-Lymphocyte Ratio as an Inflammatory Marker in Rheumatic Diseases. Ann. Lab. Med. 2019 Jul. 39(4). 345-357. doi: 10.3343/alm.2019.39.4.345.
- Bilgin S., Aktas G., Zahid Kocak M., Atak B.M., Kurtkulagi O., Duman T.T., Savli H. Association between novel inflammatory markers derived from hemogram indices and metabolic parameters in type 2 diabetic men. Aging Male. 2020. 23(5). 923-927. doi: 10.1080/13685538.2019.1632283.
- Ji S., Ning X., Zhang B., Shi H., Liu Z., Zhang J. Platelet distribution width, platelet count, and plateletcrit in diabetic retinopathy: A systematic review and meta-analysis of PRISMA guidelines. Medicine (Baltimore). 2019 Jul. 98(29). e16510. doi: 10.1097/MD.0000000000016510.
- Bilgin S., Aktas G., Kurtkulagi O. et al. Edmonton frail score is associated with diabetic control in elderly type 2 diabetic subjects. Journal of Diabetes and Metabolic Disorders. 2020. 19. 511-514. doi: 10.1007/s40200-020-00542-z.
- Kocak M.Z., Aktas G., Duman T.T. et al. Monocyte lymphocyte ratio As a predictor of Diabetic Kidney Injury in type 2 Diabetes mellitus; The MADKID Study. Journal of Diabetes and Metabolic Disorders. 2020. 19. 997-1002. doi: 10.1007/s40200-020-00595-0.
- Kucuk H., Tecer D., Goker B., Varan O., Babaoglu H., Guven S.C., Ozturk M.A., Haznedaroglu S., Tufan A. Platelet/lymphocyte ratio and mean platelet volume in patients with granulomatosis with polyangiitis. Adv. Rheumatol. 2019 Dec 31. 60(1). 4. doi: 10.1186/s42358-019-0110-8. PMID: 31892347.
- Bilgin S., Kurtkulagi O., Atak Tel B.M. et al. Does C-reactive protein to serum Albumin Ratio correlate with diabEtic nephropathy in patients with Type 2 dIabetes MEllitus? The CARE TIME study. Primary Care Diabetes. 2021. 15. 1071-1074. doi: 10.1016/j.pcd.2021.08.015.
- Perry R.J., Shulman G.I. Sodium-glucose cotransporter-2 inhibitors: Understanding the mechanisms for therapeutic promise and persisting risks. J. Biol. Chem. 2020 Oct 16. 295(42). 14379-14390. doi: 10.1074/jbc.REV120.008387.
- Leandro A., Queiroz M., Azul L., Seiça R., Sena C.M. Omentin: A novel therapeutic approach for the treatment of endothelial dysfunction in type 2 diabetes. Free Radic Biol. Med. 2021 Jan. 162. 233-242. doi: 10.1016/j.freeradbiomed.2020.10.021.
- Tutunchi H., Ostadrahimi A., Hosseinzadeh-Attar M.J., Miryan M., Mobasseri M., Ebrahimi-Mameghani M. A systematic review of the association of neuregulin 4, a brown fat-enriched secreted factor, with obesity and related metabolic disturbances. Obes. Rev. 2020 Feb. 21(2). e12952. doi: 10.1111/obr.12952.
- Kocak M.Z., Aktas G., Atak B.M. et al. Is Neuregulin-4 a predictive marker of microvascular complications in type 2 diabetes mellitus? European Journal of Clinical Investigation. 2020. 50. e13206. doi: 10.1111/eci.13206.
- López-Yoldi M., Moreno-Aliaga M.J., Bustos M. Cardiotrophin-1: A multifaceted cytokine. Cytokine Growth Factor Rev. 2015 Oct. 26(5). 523-32. doi: 10.1016/j.cytogfr.2015.07.009. Epub 2015 Jul 4. PMID: 26188636.
- Aktas G., Kocak M.Z., Bilgin S. et al. Uric acid to HDL cholesterol ratio is a strong predictor of diabetic control in men with type 2 diabetes mellitus. The Aging Male: the official journal of the International Society for the Study of the Aging Male. 2020. 23. 1098-1102. doi: 10.1080/13685538.2019.1678126.
- Kocak M.Z., Aktas G., Erkus E. et al. Serum uric acid to HDL-cholesterol ratio is a strong predictor of metabolic syndrome in type 2 diabetes mellitus. Revista da Associacao Medica Brasileira. 2019. 65. 9-15. doi: 10.1590/1806-9282.65.1.9.
- Chino Y., Samukawa Y., Sakai S. et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharmaceutics & Drug Disposition. 2014. 35. 391-404. doi: 10.1002/bdd.1909.
- Ouchi M., Oba K., Kaku K., Suganami H., Yoshida A., Fukunaka Y., Jutabha P., et al. Uric acid lowering in relation to HbA1c reductions with the SGLT2 inhibitor tofogliflozin. Diabetes Obes. Metab. 2018. 20(4). 1061-1065. doi: 10.1111/dom.13170.
- Devineni D., Morrow L., Hompesch M., Skee D., Vandebosch A., Murphy J., Ways K., Schwartz S. Canagliflozin improves glycaemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes. Metab. 2012. 14(6). 539-45. doi: 10.1111/j.1463-1326.2012.01558.x.
- Itani T., Ishihara T. Efficacy of canagliflozin against nonalcoholic fatty liver disease: a prospective cohort study. Obes. Sci. Pract. 2018. 4(5). 477-482. doi: 10.1002/osp4.294.

/8.jpg)