Международный эндокринологический журнал Том 18, №6, 2022
Вернуться к номеру
Метаболічний синдром і підходи до його лікування (послідовна терапія): огляд літератури
Авторы: Jitender Sorout, Sudhanshu Kacker, Neha Saboo
Department of Physiology, RUHS College of Medical Sciences, Jaipur, India
Рубрики: Эндокринология
Разделы: Справочник специалиста
Версия для печати
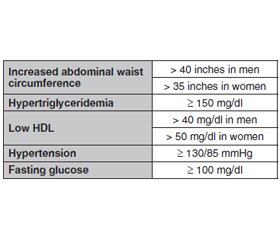
Метаболічний синдром (МС) визначається кластером факторів ризику, включаючи резистентність до інсуліну, артеріальну гіпертензію, дисліпідемію та ожиріння. Метаболічний синдром також визначається як наявність щонайменше трьох метаболічних факторів ризику — підвищеного артеріального тиску, підвищеного рівня цукру в крові, надлишку жиру в організмі та аномального рівня холестерину — і значно підвищує ймовірність майбутніх серцево-судинних проблем. За останні 50 років спостерігалося різке зростання метаболічних розладів, включаючи ожиріння та цукровий діабет (ЦД) 2-го типу, причому очікується, що до 2030 року кількість людей, у яких діагностовано ЦД 2-го типу, у всьому світі перевищить 360 мільйонів. Рання діагностика важлива для ефективного використання способу життя та модифікації фактора ризику. Медикаментозна терапія МС спрямована на лікування окремих компонентів МС, зокрема використання антигіпертензивних засобів, статинів та метформіну, деяких природних сполук, методів йоги та дотримання способу життя. Тому в цій оглядовій статті розглянуті різні методи лікування. Світ гостро потребує пошуку методів лікування метаболічного синдрому. Інсулінорезистентність і дисліпідемія відіграють центральну роль у патофізіології цього синдрому. У сучасному світі метаболічний синдром набуває масштабів епідемії. З огляду на те, що лише невелика кількість людей дотримується здорового харчування та способу життя, більшість все ще потрапляє в групу тих, хто не дотримується належного способу життя. Соціально-економічні зміни та, зрештою, глобалізація призвели до трансформації суспільства. Це призвело до змін у харчових звичках, що зрештою призвело до зміни режиму харчування. Медикаментозне лікування ґрунтується лише на корекції певних симптомів. Нещодавно схвалені препарати проти ожиріння можна призначати для зменшення маси тіла, зокрема абдомінального, вісцерального жиру. Втручання першої лінії, націлене на МС, передбачає зміну дієти та способу життя з регулярною фізичною активністю протягом певного періоду часу. Однак покращення параметрів MС можна спостерігати лише в тих випадках, коли ці модифікації підтримуються впродовж тривалого часу. Тому для цілісного подолання МС необхідна безперервна зміна дієти та способу життя. Основним лікуванням метаболічного синдрому є усунення причинних факторів ризику його розвитку.
Metabolic syndrome (MS) is defined by a cluster of risk factors including insulin resistance, hypertension, dyslipidemia, and obesity. Metabolic syndrome is also defined as having at least three metabolic risk factors — increased blood pressure, high blood sugar level, excess body fat, and abnormal cholesterol levels — and greatly increases the chance of future cardiovascular problems. The last 50 years have seen a dramatic increase in metabolic disorders, including obesity and type 2 diabetes, with the number of individuals diagnosed with type 2 diabetes worldwide expected to surpass 360 million by 2030. Early diagnosis is important in order to employ effectively lifestyle and risk factor modification. Pharmaceutical therapy in MS is aimed at treating the individual components of MS such as antihypertensives, statins, and metformin. Some natural compounds, Yoga and dietary elements. Therefore in this article various therapies (possible treatments) were reviewed. The world is in emergent need for searching of treatments for metabolic syndrome. The MS is a constellation of common metabolic disorders that is associated with type 2 diabetes and cardiovascular disease. Insulin resistance and dyslipidemia play central roles in the pathophysiology of this syndrome. In this modern world, metabolic syndrome is reaching epidemic proportions. With only a handful of people following the healthy diet and lifestyle, majority still fall in the bracket of those with compromised diet and lifestyle, burdening the health services. Socio economic changes and eventually globalization has led to transformation in the society. This has led to alterations in the dietary habits eventually resulting in nutrition transition. Pharmacological treatment is only based on only diagnosis and symptoms. Recently approved anti-obesity drugs can be prescribed to reduce body weight, particularly abdominal visceral fat. A first line intervention targeting MS involves dietary and lifestyle modification with regular physical activity over a period of time. However, improvement in MS parameters can only be maintained when these modifications can be sustained. Therefore, dietary and lifestyle modification in continuum is required to overcome MS holistically. The main and foremost treatment for metabolic syndrome is to eliminate the causative risk factors of it.
цукровий діабет; хронічні неінфекційні захворювання; лікування; метаболічний синдром
diabetes; non communicable diseases; treatment and metabolic syndrome
Introduction
/48.jpg)
Possible treatments of Metabolic Syndrome Lifestyle modification
Yoga
Conclusions
- Mohan P., Mohan S.B., Dutta M. Communicable or noncommunicable diseases? Building strong primary health care systems to address double burden of disease in India. J. Family Med. Prim. Care. 2019. 8(2). 326-329. doi: 10.4103/jfmpc.jfmpc_67_19. PMID: 30984632; PMCID: PMC6436242.
- GBD 2019 Ageing Collaborators. Global, regional, and national burden of diseases and injuries for adults 70 years and older: systematic analysis for the Global Burden of Disease 2019 Study. BMJ. 2022. 376. e068208. doi: 10.1136/bmj-2021-068208. PMID: 35273014; PMCID: PMC9316948.
- Khan M.A.B., Hashim M.J., King J.K., Govender R.D., Mustafa H., Al Kaabi J. Epidemiology of Type 2 Diabetes — Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020. 10(1). 107-111. doi: 10.2991/jegh.k.191028.001. PMID: 32175717; PMCID: PMC7310804.
- Standl E., Khunti K., Hansen T.B., Schnell O. The global epidemics of diabetes in the 21st century: Current situation and perspectives. Eur. J. Prev. Cardiol. 2019. 26(2_suppl.). 7-14. doi: 10.1177/2047487319881021. PMID: 31766915.
- Miller J.M., Kaylor M.B., Johannsson M., Bay C., Churilla J.R. Prevalence of metabolic syndrome and individual criterion in US adolescents: 2001-2010 National Health and Nutrition Examination Survey. Metab. Syndr. Relat. Disord. 2014. 12(10). 527-32. doi: 10.1089/met.2014.0055.
- Khatri P.M., Modi V. Prevalence of metabolic syndrome in higher socioeconomic class of Bikaner, Rajasthan, India. IJSR. 2018. 7(7). 48-50. DOI: 10.36106/ijsr.
- Bovolini A., Garcia J., Andrade M.A., Duarte J.A. Metabolic Syndrome Pathophysiology and Predisposing Factors. Int. J. Sports Med. 2021. 42(3). 199-214. doi: 10.1055/a-1263-0898.
- Fahed G., Aoun L., Bou Zerdan M., Allam S., Bou Zerdan M., Bouferraa Y., Assi H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022. 23(2). 786. doi: 10.3390/ijms23020786. PMID: 35054972; PMCID: PMC8775991.
- Regufe V.M.G., Pinto C.M.C.B., Perez P.M.V.H.C. Metabolic syndrome in type 2 diabetic patients: a review of current evidence. Porto Biomed. J. 2020. 5(6). e101. doi: 10.1097/j.pbj.0000000000000101. PMID: 33299950; PMCID: PMC7721212.
- Shin J.A., Lee J.H., Lim S.Y., Ha H.S., Kwon H.S., Park Y.M., Lee W.C. et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J. Diabetes Investig. 2013. 4(4). 334-43. doi: 10.1111/jdi.12075.
- Tune J.D., Goodwill A.G., Sassoon D.J., Mather K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017. 183. 57-70. doi: 10.1016/j.trsl.2017.01.001.
- Kuo W.C., Bratzke L.C., Oakley L.D., Kuo F., Wang H., Brown R.L. The association between psychological stress and metabolic syndrome: A systematic review and meta-analysis. Obes. Rev. 2019. 20(11). 1651-1664. doi: 10.1111/obr.12915.
- Bergmann N., Gyntelberg F., Faber J. The appraisal of chronic stress and the development of the metabolic syndrome: a systematic review of prospective cohort studies. Endocr. Connect. 2014. 3(2). 55-80. doi: 10.1530/EC-14-0031.
- Fabiani R., Naldini G., Chiavarini M. Dietary Patterns and Metabolic Syndrome in Adult Subjects: A Systematic Review and Meta-Analysis. Nutrients. 2019. 11(9). 2056. doi: 10.3390/nu11092056. PMID: 31480732; PMCID: PMC6770202.
- Kolovou G.D., Anagnostopoulou K.K., Salpea K.D., Mikhailidis D.P. The prevalence of metabolic syndrome in various populations. Am. J. Med. Sci. 2007. 333(6). 362-71. doi: 10.1097/MAJ.0b013e318065c3a1. PMID: 17570989.
- Cameron A.J., Shaw J.E., Zimmet P.Z. The metabolic syndrome: prevalence in worldwide populations. Endocrinol. Metab. Clin. North. Am. 2004. 33(2). 351-75, table of contents. doi: 10.1016/j.ecl.2004.03.005. PMID: 15158523.
- Park Y.W., Zhu S., Palaniappan L., Heshka S., Carnethon M.R., Heymsfield S.B. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch. Intern. Med. 2003. 163(4). 427-36. doi: 10.1001/archinte.163.4.427. PMID: 12588201; PMCID: PMC3146257.
- Palaniappan L., Carnethon M.R., Wang Y., Hanley A.J., Fortmann S.P., Haffner S.M., Wagenknecht L.; Insulin Resistance Atherosclerosis Study. Predictors of the incident metabolic syndrome in adults: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2004. 27(3). 788-93. doi: 10.2337/diacare.27.3.788. PMID: 14988303.
- Krishnamoorthy Y., Rajaa S., Murali S., Rehman T., Sahoo J., Kar S.S. Prevalence of metabolic syndrome among adult population in India: A systematic review and meta-analysis. PLoS One. 2020. 15(10). e0240971. doi: 10.1371/journal.pone.0240971. PMID: 33075086; PMCID: PMC7571716.
- Beilby J. Definition of Metabolic Syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Clin. Biochem. Rev. 2004. 25(3). 195-8. PMCID: PMC1880831.
- Paley C.A., Johnson M.I. Abdominal obesity and metabolic syndrome: exercise as medicine? BMC Sports Sci. Med. Rehabil. 2018. 10. 7. doi: 10.1186/s13102-018-0097-1. PMID: 29755739; PMCID: PMC5935926.
- Gustat J., Srinivasan S.R., Elkasabany A., Berenson G.S. Relation of self-rated measures of physical activity to multiple risk factors of insulin resistance syndrome in young adults: the Bogalusa Heart Study. J. Clin. Epidemiol. 2002. 55(10). 997-1006. doi: 10.1016/s0895-4356(02)00427-4. PMID: 12464376.
- Cárdenas Fuentes G., Bawaked R.A., Martínez González M.Á., Corella D., Subirana Cachinero I., Salas-Salvadó J., Estruch R. et al. Association of physical activity with body mass index, waist circumference and incidence of obesity in older adults. Eur. J. Public. Health. 2018. 28(5). 944-950. doi: 10.1093/eurpub/cky030. PMID: 29554269.
- Föhr T., Pietilä J., Helander E., Myllymäki T., Lindholm H., Rusko H., Kujala U.M. Physical activity, body mass index and heart rate variability-based stress and recovery in 16 275 Finnish employees: a cross-sectional study. BMC Public Health. 2016. 16. 701. doi: 10.1186/s12889-016-3391-4. PMID: 27484470; PMCID: PMC4971625.
- Ofori E.K., Angmorterh S.K. Relationship between physical activity, body mass index (BMI) and lipid profile of students in Ghana. Pan Afr. Med. J. 2019. 33. 30. doi: 10.11604/pamj.2019.33.30.17889. PMID: 31384345; PMCID: PMC6658156.
- Wallace M.K., Shivappa N., Wirth M.D., Hébert J.R., Huston-Gordesky L., Alvarado F., Mouzon S.H., Catalano P.M. Longitudinal Assessment of Relationships Between Health Behaviors and IL-6 in Overweight and Obese Pregnancy. Biol. Res. Nurs. 2021. 23(3). 481-487. doi: 10.1177/1099800420985615.
- Rehman K., Akash M.S.H., Liaqat A., Kamal S., Qadir M.I., Rasul A. Role of Interleukin-6 in Development of Insulin Resistance and Type 2 Diabetes Mellitus. Crit. Rev. Eukaryot. Gene Expr. 2017. 27(3). 229-236. doi: 10.1615/CritRevEukaryotGeneExpr.2017019712. PMID: 29199608.
- Harder-Lauridsen N.M., Krogh-Madsen R., Holst J.J., Plomgaard P., Leick L., Pedersen B.K., Fischer C.P. Effect of IL-6 on the insulin sensitivity in patients with type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2014. 306(7). 769-78. doi: 10.1152/ajpendo.00571.2013.
- Nieto-Vazquez I., Fernández-Veledo S., Krämer D.K., Vila-Bedmar R., Garcia-Guerra L., Lorenzo M. Insulin resistance associated to obesity: the link TNF-alpha. Arch. Physiol. Biochem. 2008. 114(3). 183-94. doi: 10.1080/13813450802181047.
- Yang X., Ongusaha P.P., Miles P.D., Havstad J.C., Zhang F., So W.V., Kudlow J.E. et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008. 451(7181). 964-9. doi: 10.1038/nature06668. PMID: 18288188.
- Zhou B., Zhang Y., Li S., Wu L., Fejes-Toth G., Naray-Fejes-Toth A., Soukas A.A. Serum- and glucocorticoid-induced kinase drives hepatic insulin resistance by directly inhibiting AMP-activated protein kinase. Cell. Rep. 2021. 37(1). 109785. doi: 10.1016/j.celrep.2021.109785. PMID: 34610303; PMCID: PMC8576737.
- Ruderman N.B., Carling D., Prentki M., Cacicedo J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Invest. 2013. 123(7). 2764-72. doi: 10.1172/JCI67227.
- Dutta A., Aruchunan M., Mukherjee A., Metri K.G., Ghosh K., Basu-Ray I. A Comprehensive Review of Yoga Research in 2020. J. Integr. Complement. Med. 2022. 28(2). 114-123. doi: 10.1089/jicm.2021.0420.
- Cramer H., Lauche R., Anheyer D., Pilkington K., de Manincor M., Dobos G., Ward L. Yoga for anxiety: A systematic review and meta-analysis of randomized controlled trials. Depress Anxiety. 2018. 35(9). 830-843. doi: 10.1002/da.22762.
- Yadav R., Yadav R.K., Pandey R.M., Kochar K.P. Effect of a Short-Term Yoga-Based Lifestyle Intervention on Health-Related Quality of Life in Overweight and Obese Subjects. J. Altern. Complement. Med. 2016. 22(6). 443-9. doi: 10.1089/acm.2015.0268.
- Siu P.M., Yu A.P., Benzie I.F. et al. Effects of 1-year yoga on cardiovascular risk factors in middle-aged and older adults with metabolic syndrome: a randomized trial. Diabetol. Metab. Syndr. 2015. 7. 40. https://doi.org/10.1186/s13098-015-0034-3.
- Lee J.A., Kim J.W., Kim D.Y. Effects of yoga exercise on serum adiponectin and metabolic syndrome factors in obese postmenopausal women. Menopause. 2012. 19(3). 296-301. doi: 10.1097/gme.0b013e31822d59a2.
- Nagarathna R., Tyagi R., Kaur G., Vendan V., Acharya I.N., Anand A., Singh A., Nagendra H.R. Efficacy of a Validated Yoga Protocol on Dyslipidemia in Diabetes Patients: NMB-2017 India Trial. Medicines (Basel). 2019. 6(4). 100. doi: 10.3390/medicines6040100. PMID: 31614579; PMCID: PMC6963794.
- Yu A.P., Ugwu F.N., Tam B.T., Lee P.H., Lai C.W., Wong C.S.C., Lam W.W., Sheridan S., Siu P.M. One Year of Yoga Training Alters Ghrelin Axis in Centrally Obese Adults With Metabolic Syndrome. Front Physiol. 2018. 9. 1321. doi: 10.3389/fphys.2018.01321. PMID: 30294284; PMCID: PMC6158302.
- Tikhe A.S., Pailoor S., Metri K., Ganpat T.S., Ramarao N.H. Yoga: Managing overweight in mid-life T2DM. J. Midlife Health. 2015. 6(2). 81-4. doi: 10.4103/0976-7800.158959. PMID: 26167059; PMCID: PMC4481745.
- Raveendran A.V., Deshpandae A., Joshi S.R. Therapeutic Role of Yoga in Type 2 Diabetes. Endocrinol. Metab. (Seoul). 2018. 33(3). 307-317. doi: 10.3803/EnM.2018.33.3.307.
- Shantakumari N., Sequeira S., El deeb R. Effects of a yoga intervention on lipid profiles of diabetes patients with dyslipidemia. Indian. Heart J. 2013. 65(2). 127-31. doi: 10.1016/j.ihj.2013.02.010.
- Vijayaraghava A., Doreswamy V., Narasipur O.S., Kunnavil R., Srinivasamurthy N. Effect of Yoga Practice on Levels of Inflammatory Markers After Moderate and Strenuous Exercise. J. Clin. Diagn. Res. 2015. 9(6). CC08-12. doi: 10.7860/JCDR/2015/12851.6021.
- Kiecolt-Glaser J.K., Christian L.M., Andridge R., Hwang B.S., Malarkey W.B., Belury M.A., Emery C.F., Glaser R. Adiponectin, leptin, and yoga practice. Physiol. Behav. 2012. 107(5). 809-13. doi: 10.1016/j.physbeh.2012.01.016.
- Janochova K., Haluzik M., Buzga M. Visceral fat and insulin resistance — what we know? Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2019. 163(1). 19-27. doi: 10.5507/bp.2018.062.
- Afonso R.F., Kozasa E.H., Rodrigues D., Leite J.R., Tufik S., Hachul H. Yoga increased serum estrogen levels in postmenopausal women-a case report. Menopause. 2016. 23(5). 584-6. doi: 10.1097/GME.0000000000000593. PMID: 26926324.
- Lemay V., Hoolahan J., Buchanan A. Impact of a Yoga and Meditation Intervention on Students' Stress and Anxiety Levels. Am. J. Pharm. Educ. 2019. 83(5). 7001. doi: 10.5688/ajpe7001. PMID: 31333265; PMCID: PMC6630857.
- McCall T. The Scientific Basis of Yoga Therapy. [Accessed December 07, 2021]. At http://www.yogajournal.com/for_teachers/2016 .
- Chandratreya S. Diabetes & Yoga. [Accessed December 7, 2021] at http://www.yogapoint.com/therapy/diabetes_yoga.htm.
- Cramer H., Lauche R., Haller H., Dobos G., Michalsen A. A systematic review of yoga for heart disease. Eur. J. Prev. Cardiol. 2015. 22(3). 284-95. doi: 10.1177/2047487314523132.
- Jayawardena R., Ranasinghe P., Ranawaka H., Gamage N., Dissanayake D., Misra A. Exploring the Therapeutic Benefits of Pranayama (Yogic Breathing): A Systematic Review. Int. J. Yoga. 2020. 13(2). 99-110. doi: 10.4103/ijoy.IJOY_37_19.
- Jevning R., Wallace R.K., Beidebach M. The physiology of meditation: a review. A wakeful hypometabolic integrated response. Neurosci. Biobehav. Rev. 1992. 16(3). 415-24. doi: 10.1016/s0149-7634(05)80210-6. PMID: 1528528.
- Mozaffarian D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation. 2016. 133(2). 187-225. doi: 10.1161/CIRCULATIONAHA. 115.018585. PMID: 26746178; PMCID: PMC4814348.
- Al-Hadlaq S.M., Balto H.A., Hassan W.M., Marraiki N.A., El-Ansary A.K. Biomarkers of non-communicable chronic disease: an update on contemporary methods. Peer J. 2022. 10. e12977. doi: 10.7717/peerj.12977. PMID: 35233297; PMCID: PMC8882335.
- Steckhan N., Hohmann C.D., Kessler C., Dobos G., Michalsen A., Cramer H. Effects of different dietary approaches on inflammatory markers in patients with metabolic syndrome: A systematic review and meta-analysis. Nutrition. 2016. 32. 338-348. https://doi.org/10.1016/j.nut.2015.09.010
- Godos J., Zappalà G., Bernardini S., Giambini I., Bes-Rastrollo M., Martinez-Gonzalez M. Adherence to the Mediterranean diet is inversely associated with metabolic syndrome occurrence: a meta-analysis of observational studies. Int. J. Food Sci. Nutr. 2017. 68(2). 138-148. doi: 10.1080/09637486.2016.1221900.
- Landaeta-Díaz L., Fernández J.M., Da Silva-Grigoletto M., Rosado-Alvarez D., Gómez-Garduño A., Gómez-Delgado F., López-Miranda J., Pérez-Jiménez F., Fuentes-Jiménez F. Mediterranean diet, moderate-to-high intensity training, and health-related quality of life in adults with metabolic syndrome. Eur. J. Prev. Cardiol. 2013. 20(4). 555-64. doi: 10.1177/2047487312445000.
- Bahr L.S., Franz K., Mähler A. Assessing the (anti)-inflammatory potential of diets. Curr. Opin. Clin. Nutr. Metab. Care. 2021. 24(5). 402-410. doi: 10.1097/MCO.0000000000000772. PMID: 34155152.
- Franquesa M., Pujol-Busquets G., García-Fernández E., Rico L., Shamirian-Pulido L., Aguilar-Martínez A., Medina F.X. et al. Mediterranean Diet and Cardiodiabesity: A Systematic Review through Evidence-Based Answers to Key Clinical Questions. Nutrients. 2019. 11(3). 655. doi: 10.3390/nu11030655. PMID: 30889891; PMCID: PMC6471908.
- Shenoy S.F., Poston W.S., Reeves R.S., Kazaks A.G., Holt R.R., Keen C.L., Chen H.J. et al. Weight loss in individuals with metabolic syndrome given DASH diet counseling when provided a low sodium vegetable juice: a randomized controlled trial. Nutr. J. 2010. 9. 8. doi: 10.1186/1475-2891-9-8. PMID: 20178625; PMCID: PMC2841082.
- Muzio F., Mondazzi L., Harris W.S., Sommariva D., Branchi A. Effects of moderate variations in the macronutrient content of the diet on cardiovascular disease risk factors in obese patients with the metabolic syndrome. Am. J. Clin. Nutr. 2007. 86(4). 946-51. doi: 10.1093/ajcn/86.4.946. PMID: 17921369.
- Rask Larsen J., Dima L., Correll C.U., Manu P. The pharmacological management of metabolic syndrome. Expert Rev. Clin. Pharmacol. 2018. 11(4). 397-410. doi: 10.1080/17512433.2018. 1429910.
- Stemmer K., Müller T.D., DiMarchi R.D., Pfluger P.T., Tschöp M.H. CNS-targeting pharmacological interventions for the metabolic syndrome. J. Clin. Invest. 2019. 129(10). 4058-4071. doi: 10.1172/JCI129195. PMID: 31380808; PMCID: PMC6763237.
- Tabrizi R., Tamtaji O.R., Mirhosseini N., Lankarani K.B., Akbari M., Dadgostar E., Borhani-Haghighi A., Peymani P., Ahmadizar F., Asemi Z. The effects of statin use on inflammatory markers among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019. 141. 85-103. doi: 10.1016/j.phrs.2018.12.010.
- Kim N.H., Han K.H., Choi J., Lee J., Kim S.G. Use of fenofibrate on cardiovascular outcomes in statin users with metabolic syndrome: propensity matched cohort study. BMJ. 2019. 366. l5125. doi: 10.1136/bmj.l5125. PMID: 31562117; PMCID: PMC6763755.
- Ohbu-Murayama K., Adachi H., Hirai Y., Enomoto M., Fukami A., Obuchi A., Yoshimura A. et al. Ezetimibe combined with standard diet and exercise therapy improves insulin resistance and atherosclerotic markers in patients with metabolic syndrome. J. Diabetes Investig. 2015. 6(3). 325-33. doi: 10.1111/jdi.12298.
- Adiels M., Chapman M.J., Robillard P., Krempf M., Laville M., Borén J.; Niacin Study Group. Niacin action in the atherogenic mixed dyslipidemia of metabolic syndrome: Insights from metabolic biomarker profiling and network analysis. J. Clin. Lipidol. 2018. 12(3). 810-821.e1. doi: 10.1016/j.jacl.2018.03.083.
- Vega G.L., Wang J., Grundy S.M. Utility of metabolic syndrome as a risk enhancing factor in decision of statin use. J. Clin. Lipidol. 2021. 15(2). 255-265. doi: 10.1016/j.jacl.2021.01.012.
- Sugizaki T., Watanabe M., Horai Y., Kaneko-Iwasaki N., Arita E., Miyazaki T., Morimoto K., Honda A., Irie J., Itoh H. The Niemann-Pick C1 like 1 (NPC1L1) inhibitor ezetimibe improves metabolic disease via decreased liver X receptor (LXR) activity in liver of obese male mice. Endocrinology. 2014. 155(8). 2810-9. doi: 10.1210/en.2013-2143.
- Nauck M.A., Wefers J., Meier J.J. Treatment of type 2 diabetes: challenges, hopes, and anticipated successes. Lancet Diabetes Endocrinol. 2021. 9(8). 525-544. doi: 10.1016/S2213-8587(21)00113-3.
- Borse S.P., Chhipa A.S., Sharma V., Singh D.P., Nivsarkar M. Management of Type 2 Diabetes: Current Strategies, Unfocussed Aspects, Challenges, and Alternatives. Med. Princ. Pract. 2021. 30(2). 109-121. doi: 10.1159/000511002.