Международный эндокринологический журнал Том 19, №3, 2023
Вернуться к номеру
Відоме та невідоме про вроджений гіпотиреоз: оновлення за 2022 рік
Авторы: Tomoyo Itonaga (1), Yukihiro Hasegawa (2), Shinji Higuchi (3), Mari Satoh (4), Hirotake Sawada (5), Kazuhiro Shimura (6), Ikuko Takahashi (7), Noriyuki Takubo (8), Keisuke Nagasaki (9)
(1) — Department of Pediatrics, Oita University Faculty of Medicine, Oita, Japan
(2) — Division of Endocrinology and Metabolism, Tokyo Metropolitan Children’s Medical Center, Tokyo, Japan
(3) — Division of Pediatric Endocrinology and Metabolism, Children’s Medical Center, Osaka City General Hospital, Osaka, Japan
(4) — Department of Pediatrics, Toho University Omori Medical Center, Tokyo, Japan
(5) — Division of Pediatrics, Faculty of Medicine, University of Miyazaki Hospital, Miyazaki, Japan
(6) — Department of Pediatrics, Keio University School of Medicine, Tokyo, Japan
(7) — Department of Pediatrics, Akita University Graduate School of Medicine, Akita, Japan
(8) — Department of Pediatrics and Adolescent Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan
(9) — Department of Pediatrics, Niigata University Medical and Dental Hospital, Niigata, Japan
Рубрики: Эндокринология
Разделы: Справочник специалиста
Версия для печати
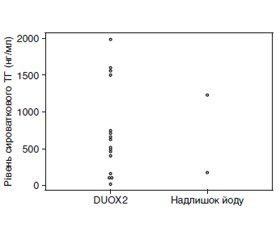
На сьогодні доступні деякі високоякісні рекомендації та експертні думки щодо вродженого гіпотиреозу (ВГ). Однак деякі моменти, пов’язані з ВГ, у них не розглядаються детально. У даному огляді автори обговорюють сім клінічних питань, які, на їхню думку, є особливо важливими, сподіваючись, що привернення уваги до них допоможе дитячим ендокринологам у подальшому лікуванні ВГ. 1. Наскільки високою має бути доза левотироксину (L-T4) для початкового лікування тяжкого і перманентного ВГ? 2. Який оптимальний метод моніторингу лікування тяжкого ВГ? 3. Яка доза споживання йоду матір’ю під час вагітності впливає на функцію щитоподібної залози плода та новонародженого? 4. Чи відрізняється сироватковий тиреоглобулін у пацієнтів з варіантами подвійної оксидази 2 (DUOX2) і в пацієнтів з надлишком йоду? 5. Кому треба проводити генетичну діагностику? 6. Який показник є найкращим для того, щоб розрізнити транзиторний і перманентний ВГ? 7. Чи існує ризик раку, пов’язаний із ВГ? Автори обговорили ці теми та разом написали цей огляд, щоб поліпшити розуміння ВГ і пов’язаних з ним питань.
Several excellent guidelines and expert opinions on congenital hypothyroidism (CH) are currently available. Nonetheless, these guidelines do not address several issues related to CH in detail. In this review, the authors chose the following seven clinical issues that they felt were especially deserving of closer scrutiny in the hope that drawing attention to them through discussion would help pediatric endocrinologists and promote further interest in the treatment of CH. 1. How high should the levothyroxine (L-T4) dose be for initial treatment of severe and permanent CH? 2. What is the optimal method for monitoring treatment of severe CH? 3. At what level does maternal iodine intake during pregnancy affect fetal and neonatal thyroid function? 4. Does serum thyroglobulin differ between patients with a dual oxidase 2 (DUOX2) variants and those with excess iodine? 5. Who qualifies for a genetic diagnosis? 6. What is the best index for distinguishing transient and permanent CH? 7. Is there any cancer risk associated with CH? The authors discussed these topics and jointly edited the manuscript to improve the understanding of CH and related issues.
вроджений гіпотиреоз; подвійна оксидаза 2 (DUOX2); йод; левотироксин; молекулярна генетика
congenital hypothyroidism; the dual oxidase 2 (DUOX2); iodine; levothyroxine; molecular genetics
Вступ
/80.jpg)
- Rose S.R., Brown R.S., Foley T., Kaplowitz P.B., Kaye C.I., Sundararajan S. et al. American Academy of Pediatrics Section on Endocrinology and Committee on Genetics, American Thyroid Associa–tion Public Health Committee, Lawson Wilkins Pediatric Endocrine Society. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics. 2006. 117. 2290-303.
- Leger J., Olivieri A., Donaldson M., Torresani T., Krude H., van Vliet G. et al. ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE Congenital Hypothyroidism Consensus Conference Group. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J. Clin. Endocrinol. Metab. 2014. 99. 363-84.
- Nagasaki K., Minamitani K., Anzo M., Adachi M., Ishii T., Onigata K. et al. Mass Screening Committee Japanese Society for Pediatric Endocrinology Japanese Society for Mass Screening. Guidelines for mass screening of congenital hypothyroidism (2014 revision). Clin. Pediatr. Endocrinol. 2015. 24. 107-33.
- van Trotsenburg P., Stoupa A., Leger J., Rohrer T., Peters C., Fugazzola L. et al. Congenital hypothyroidism: a 2020–2021 consensus guidelines update-an ENDO-European Reference Network Initiative Endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid. 2021. 31. 387-419.
- van der Sluijs Veer L., Kempers M.J., Wiedijk B.M., Last B.F., Grootenhuis M.A., Vulsma T. Evaluation of cognitive and motor development in toddlers with congenital hypothyroidism diagnosed by neonatal screening. J. Dev. Behav. Pediatr. 2012. 33. 633-40. [Medline] [CrossRef]
- Ng S.M., Anand D., Weindling A.M. High versus low dose of initial thyroid hormone replacement for congenital hypothyroidism. Cochrane Database Syst. Rev. 2009. CD006972.
- Dubuis J.M., Glorieux J., Richer F., Deal C.L., Dussault J.H., Van Vliet G. Outcome of severe congenital hypothyroidism: closing the developmental gap with early high dose levothyroxine treatment. J. Clin. Endocrinol. Metab. 1996. 81. 222-7. [Medline]
- Bongers-Schokking J.J., Koot H.M., Wiersma D., Verkerk P.H., de Muinck Keizer-Schrama S.M. Influence of timing and dose of thyroid hormone replacement on development in infants with congenital hypothyroidism. J. Pediatr. 2000. 136. 292-7. [Medline] [CrossRef]
- Salerno M., Militerni R., Bravaccio C., Micillo M., Capalbo D., Di M.S. et al. Effect of different starting doses of levothyroxine on growth and intellectual outcome at four years of age in congenital hypothyroidism. Thyroid. 2002. 12. 45-52. [Medline] [CrossRef]
- Dimitropoulos A., Molinari L., Etter K., Torresani T., Lang-Muritano M., Jenni O.G. et al. Children with congenital hypothyroidism: long-term intellectual outcome after early high-dose treatment. Pediatr. Res. 2009. 65. 242-8. [Medline] [CrossRef]
- Hauri-Hohl A., Dusoczky N., Dimitropoulos A., Leuchter R.H., Molinari L., Caflisch J. et al. Impaired neuromotor outcome in school-age children with congenital hypothyroidism receiving early high-dose substitution treatment. Pediatr. Res. 2011. 70. 614-8. [Medline] [CrossRef]
- Albert B.B., Heather N., Derraik J.G., Cutfield W.S., Wouldes T., Tregurtha S. et al. Neurodevelopmental and body composition outcomes in children with congenital hypothyroidism treated with high-dose initial replacement and close monitoring. J. Clin. Endocrinol. Metab. 2013. 98. 3663-70. [Medline] [CrossRef]
- Bongers-Schokking J.J., Resing W.C., de Rijke Y.B., de Ridder M.A., de Muinck Keizer-Schrama S.M. Cognitive development in congenital hypothyroidism: is overtreatment a greater threat than undertreatment? J. Clin. Endocrinol. Metab. 2013. 98. 4499-506. [Medline] [CrossRef]
- Aleksander P.E., Bruckner-Spieler M., Stoehr A.M., Lan–kes E., Kuhnen P., Schnabel D. et al. Mean high-dose l-thyroxine treatment is efficient and safe to achieve a normal IQ in young adult patients with congenital hypothyroidism. J. Clin. Endocrinol. Metab. 2018. 103. 1459-69. [Medline] [CrossRef]
- Bongers-Schokking J.J., Resing W.C., Oostdijk W., de Rijke Y.B., de Muinck Keizer-Schrama S.M. Individualized treatment to optimize eventual cognitive outcome in congenital hypothyroidism. Pediatr. Res. 2016. 80. 816-23. [Medline] [CrossRef]
- Bongers-Schokking J.J., Resing W.C.M., Oostdijk W., de Rijke Y.B., de Muinck Keizer-Schrama S.M.P.F. Relation between early over- and undertreatment and behavioural problems in preadolescent children with congenital hypothyroidism. Horm. Res. Paediatr. 2018. 90. 247-56. [Medline] [CrossRef]
- Craven M., Frank G.R. Does initial dosing of levothyroxine in infants with congenital hypothyroidism lead to frequent dose adjustments secondary to iatrogenic hyperthyroidism on follow-up? J. Pediatr. Endocrinol. Metab. 2018. 31. 597-600. [Medline] [CrossRef]
- Tuhan H., Abaci A., Cicek G., Anik A., Catli G., Demir K. et al. Levothyroxine replacement in primary congenital hypothyroidism: the higher the initial dose the higher the rate of overtreatment. J. Pe–diatr. Endocrinol. Metab. 2016. 29. 133-8. [Medline] [CrossRef]
- Vaidyanathan P., Pathak M., Kaplowitz P.B. In congenital hypothyroidism, an initial L-thyroxine dose of 10-12 pg/kg/day is sufficient and sometimes excessive based on thyroid tests 1 month later. J. Pediatr. Endocrinol. Metab. 2012. 25. 849-52. [Medline] [CrossRef]
- Uyttendaele M., Lambert S., Tenoutasse S., Boros E., Ziereisen F., Van Vliet G. et al. Congenital hypothyroidism: Long-term experience with early and high levothyroxine dosage. Horm. Res. Paediatr. 2016. 85. 188-97. [Medline] [CrossRef]
- Schneider M.J., Fiering S.N., Pallud S.E., Parlow A.F., St Germain D.L., Galton V.A. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol. Endocrinol. 2001. 15. 2137-48. [Medline] [CrossRef]
- Peeters R.P., Visser T.J. Metabolism of Thyroid Hormone. 2017 2017/01/01. In: Endotext [Internet]. MDText.com, Inc. Avai–lable from: https://www.ncbi.nlm.nih.gov/pubmed/.
- Fisher D.A., Schoen E.J., La Franchi S., Mandel S.H., Nelson J.C., Carlton E.I. et al. The hypothalamic-pituitary-thyroid negative feedback control axis in children with treated congenital hypothyroidism. J. Clin. Endocrinol. Metab. 2000. 85. 2722-7. [Medline] [CrossRef]
- Bagattini B., Cosmo C.D., Montanelli L., Piaggi P., Ciampi M., Agretti P. et al. The different requirement of L-T4 therapy in congenital athyreosis compared with adult-acquired hypothyroidism suggests a persisting thyroid hormone resistance at the hypothalamic-pituitary level. Eur. J. Endocrinol. 2014. 171. 615-21. [Medline] [CrossRef]
- Cavaliere H., Medeiros-Neto G.A., Rosner W., Kourides I.A. Persistent pituitary resistance to thyroid hormone in congenital versus later-onset hypothyroidism. J. Endocrinol. Invest. 1985. 8. 527-32. [Medline] [CrossRef]
- Wiersinga W.M. Paradigm shifts in thyroid hormone replacement therapies for hypothyroidism. Nat. Rev. Endocrinol. 2014. 10. 164-74. [Medline] [CrossRef]
- Ain K.B., Pucino F., Shiver T.M., Banks S.M. Thyroid hormone levels affected by time of blood sampling in thyroxine-treated patients. Thyroid. 1993. 3. 81-5. [Medline] [CrossRef]
- Alvarez M., Iglesias Fernandez C., Rodriguez Sanchez A., Dulin Lniguez E., Rodriguez Arnao M.D. Episodes of overtreatment during the first six months in children with congenital hypothyroidism and their relationships with sustained attention and inhibitory control at school age. Horm. Res. Paediatr. 2010. 74. 114-20. [Medline] [CrossRef]
- Ito M., Miyauchi A., Hisakado M., Yoshioka W., Ide A., Ku–do T. et al. Biochemical markers reflecting thyroid function in athyreo–tic patients on levothyroxine monotherapy. Thyroid. 2017. 27. 484-90. [Medline] [CrossRef]
- Asakura Y., Adachi M., Tachibana K. Influence of iodine excess on neonatal thyroid function (in Japanese). J. Jpn Pediatr. Soc. 2002. 106. 644-9.
- Nishiyama S., Mikeda T., Okada T., Nakamura K., Kotani T., Hishinuma A. Transient hypothyroidism or persistent hyperthyrotropinemia in neonates born to mothers with excessive iodine intake. Thyroid. 2004. 14. 1077-83. [Medline] [CrossRef]
- Theodoropoulos T., Braverman L.E., Vagenakis A.G. Iodide-induced hypothyroidism: a potential hazard during perinatal life. Science. 1979. 205. 502-3. [Medline] [CrossRef]
- Connelly K.J., Boston B.A., Pearce E.N., Sesser D., Snyder D., Braverman L.E. et al. Congenital hypothyroidism caused by excess prenatal maternal iodine ingestion. J. Pediatr. 2012. 161. 760-2. [Medline] [CrossRef]
- Markou K., Georgopoulos N., Kyriazopoulou V., Vagena–kis A.G. Iodine-Induced hypothyroidism. Thyroid. 2001. 11. 501-10. [Medline] [CrossRef]
- Lomenick J.P., Jackson W.A., Backeljauw P.F. Amiodarone-–induced neonatal hypothyroidism: a unique form of transient early-onset hypothyroidism. J. Perinatol. 2004. 24. 397-9. [Medline] [CrossRef]
- Rao R.H., McCready V.R., Spathis G.S. Iodine kinetic studies during amiodarone treatment. J. Clin. Endocrinol. Metab. 1986. 62. 563-8. [Medline] [CrossRef]
- Momotani N., Hisaoka T., Noh J., Ishikawa N., Ito K. Effects of iodine on thyroid status of fetus versus mother in treatment of Graves’ disease complicated by pregnancy. J. Clin. Endocrinol. Metab. 1992. 75. 738-44. [Medline]
- Omoto A., Kurimoto C., Minagawa M., Shozu M. A case of fetal goiter that resolved spontaneously after birth. J. Clin. Endocrinol. Metab. 2013. 98. 3910-1. [Medline] [CrossRef]
- Satoh M., Aso K., Katagiri Y. Thyroid dysfunction in neonates born to mothers who have undergone hysterosalpingography involving an oil-soluble iodinated contrast medium. Horm. Res. Paediatr. 2015. 84. 370-5. [Medline] [CrossRef]
- Sasaki Y., Kikuchi A., Murai M., Kanasugi T., Isurugi C., Oyama R. et al. Fetal goiter associated with preconception hysterosalpingography using an oil-soluble iodinated contrast medium. Ultrasound Obstet. Gynecol. 2017. 49. 275-6. [Medline] [CrossRef]
- van Welie N., Roest I., Portela M., van Rijswijk J., Koks C., Lambalk C.B. et al. H2Oil Study Group Thyroid function in neonates conceived after hysterosalpingography with iodinated contrast. Hum. Reprod. 2020. 35. 1159-67. [Medline] [CrossRef]
- Thomas J.V., Collett-Solberg P.F. Perinatal goiter with increased iodine uptake and hypothyroidism due to excess maternal iodine ingestion. Horm. Res. 2009. 72. 344-7. [Medline]
- Overcash R.T., Marc-Aurele K.L., Hull A.D., Ramos G.A. Maternal iodine exposure: a case of fetal goiter and neonatal hearing loss. Pediatrics. 2016. 137. e20153722. [Medline] [CrossRef]
- Hardley M.T., Chon A.H., Mestman J., Nguyen C.T., Geffner M.E., Chmait R.H. Iodine-induced fetal hypothyroidism: diagnosis and treatment with intra-amniotic levothyroxine. Horm. Res. Paediatr. 2018. 90. 419-23. [Medline] [CrossRef]
- Rogan W.J., Paulson J.A., Baum C., Brock-Utne A.C., Brumberg H.L., Campbell C.C. et al. Council on Environmental Health. Iodine deficiency, pollutant chemicals, and the thyroid: new information on an old problem. Pediatrics. 2014. 133. 1163-6. [Medline] [CrossRef]
- Leung A.M., Pearce E.N., Braverman L.E. Iodine content of prenatal multivitamins in the United States. N. Engl. J. Med. 2009. 360. 939-40. [Medline] [CrossRef]
- Food and Nutrition Board. 8. Iodine. In: Institute of Medicine (US) Panel on Micronutrients, editor. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academy Press; 2001. Р. 258-89.
- Study Group for dietary reference intakes. Dietary reference intakes for Japanese 2020 ver. Japan: Ministry of Health, Labor and Welfare; 2020 (in Japanese).
- Takamura N., Hamada A., Yamaguchi N., Matsushita N., Tarasiuk I., Ohashi T. et al. Urinary iodine kinetics after oral loading of potassium iodine. Endocr. J. 2003. 50. 589-93. [Medline] [CrossRef]
- Maruo Y., Takahashi H., Soeda I., Nishikura N., Matsui K., Ota Y. et al. Transient congenital hypothyroidism caused by biallelic mutations of the dual oxidase 2 gene in Japanese patients detected by a neonatal screening program. J. Clin. Endocrinol. Metab. 2008. 93. 4261-7. [Medline] [CrossRef]
- Ohye H., Fukata S., Hishinuma A., Kudo T., Nishihara E., Ito M. et al. A novel homozygous missense mutation of the dual oxidase 2 (DUOX2) gene in an adult patient with large goiter. Thyroid. 2008. 18. 561-6. [Medline] [CrossRef]
- Moreno J.C., Bikker H., Kempers M.J., van Trotsenburg A.S., Baas F., de Vijlder J.J. et al. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N. Engl. J. Med. 2002. 347. 95-102. [Medline] [CrossRef]
- Pfarr N., Korsch E., Kaspers S., Herbst A., Stach A., Zimmer C. et al. Congenital hypothyroidism caused by new mutations in the thyroid oxidase 2 (THOX2) gene. Clin. Endocrinol. (Oxf). 2006. 65. 810-5. [Medline] [CrossRef]
- Maruo Y., Nagasaki K., Matsui K., Mimura Y., Mori A., Fukami M. et al. Natural course of congenital hypothyroidism by dual oxidase 2 mutations from the neonatal period through puberty. Eur. J. Endocrinol. 2016. 174. 453-63. [Medline] [CrossRef]
- Jin H.Y., Heo S.H., Kim Y.M., Kim G.H., Choi J.H., Lee B.H. et al. High frequency of DUOX2 mutations in transient or permanent congenital hypothyroidism with eutopic thyroid glands. Horm. Res. Paediatr. 2014. 82. 252-60. [Medline] [CrossRef]
- Narumi S., Muroya K., Asakura Y., Aachi M., Hasegawa T. Molecular basis of thyroid dyshormonogenesis: genetic screening in population-based Japanese patients. J. Clin. Endocrinol. Metab. 2011. 96. E1838-42. [Medline] [CrossRef]
- Muzza M., Rabbiosi S., Vigone M.C., Zamproni I., Cirello V., Maffini M.A. et al. The clinical and molecular characterization of patients with dyshormonogenic congenital hypothyroidism reveals specific diagnostic clues for DUOX2 defects. J. Clin. Endocrinol. Metab. 2014. 99. E544-53. [Medline] [CrossRef]
- Fuse Y., Ogawa H., Fujita M., Arata N., Harada S., Oha–shi T. et al. Maternal-neonatal relationship of iodine metabolism in perinatal period: Changes in urinary iodine excretion in Japanese mothers and newborn infants. Presented at 15th International & 14th European Congress of Endocrinology. 2012. Florence, Italy. Endocrine Abstracts. 2012. 29. P1640.
- Tachibana M., Miyoshi Y., Fukui M., Onuma S., Fukuoka T., Satomura Y. et al. Urinary iodine and thyroglobulin are useful markers in infants suspected of congenital hypothyroidism based on newborn screening. Journal of pediatric endocrinology & metabolism. J. Pediatr. Endocrinol. Metab. 2021. [CrossRef]
- Narumi S., Muroya K., Asakura Y., Adachi M., Hasegawa T. Transcription factor mutations and congenital hypothyroidism: systematic genetic screening of a population-based cohort of Japanese patients. J. Clin. Endocrinol. Metab. 2010. 95. 1981-5. [Medline] [CrossRef]
- Tanaka T., Aoyama K., Suzuki A., Saitoh S., Mizuno H. Clinical and genetic investigation of 136 Japanese patients with congenital hypothyroidism. J. Pediatr. Endocrinol. Metab. 2020. 33. 691-701. [Medline] [CrossRef]
- Yamaguchi T., Nakamura A., Nakayama K., Hishimura N., Morikawa S., Ishizu K. et al. Targeted next-generation sequencing for congenital hypothyroidism with positive neonatal TSH screening. J. Clin. Endocrinol. Metab. 2020. 105. e2825-33. [Medline] [CrossRef]
- de Filippis T., Marelli F., Nebbia G., Porazzi P., Corbetta S., Fugazzola L. et al. JAG1 loss-of-function variations as a novel predisposing event in the pathogenesis of congenital thyroid defects. J. Clin. Endocrinol. Metab. 2016. 101. 861-70. [Medline] [CrossRef]
- Gannon T., Perveen R., Schlecht H., Ramsden S., Anderson B., Kerr B. et al. DDD study Further delineation of the KAT6B molecular and phenotypic spectrum. Eur. J. Hum. Genet. 2015. 23. 1165-70. [Medline] [CrossRef]
- Fu C., Luo S., Zhang Y., Fan X., D’Gama A.M., Zhang X. et al. Chromosomal microarray and whole exome sequencing identify genetic causes of congenital hypothyroidism with extra-thyroidal congenital malformations. Clin. Chim. Acta. 2019. 489. 103-8. [Medline] [CrossRef]
- Fagman H., Liao J., Westerlund J., Andersson L., Morrow B.E., Nilsson M. The 22q11 deletion syndrome candidate gene Tbx1 determines thyroid size and positioning. Hum. Mol. Genet. 2007. 16. 276-85. [Medline] [CrossRef]
- Kariyawasam D., Rachdi L., Carre A., Martin M., Hou–lier M., Janel N. et al. DYRK1A BAC transgenic mouse: a new model of thyroid dysgenesis in Down syndrome. Endocrinology. 2015. 156. 1171-80. [Medline] [CrossRef]
- de Filippis T., Gelmini G., Paraboschi E., Vigone M.C., Di Frenna M., Marelli F. et al. A frequent oligogenic involvement in congenital hypothyroidism. Hum. Mol. Genet. 2017. 26. 2507-14. [Medline] [CrossRef]
- Fan X., Fu C., Shen Y., Li C., Luo S., Li Q. et al. Next-gene–ration sequencing analysis of twelve known causative genes in congeni–tal hypothyroidism. Clin. Chim. Acta. 2017. 468. 76-80. [Medline] [CrossRef]
- Makretskaya N., Bezlepkina O., Kolodkina A., Kiyaev A., Vasilyev E.V., Petrov V. et al. High frequency of mutations in ‘dyshormonogenesis genes’ in severe congenital hypothyroidism. PLoS One. 2018. 13. e0204323. [Medline] [CrossRef]
- Peters C., van Trotsenburg A.S.P., Schoenmakers N. Diagnosis of endocrine disease: Congenital hypothyroidism: update and perspectives. Eur. J. Endocrinol. 2018. 179. R297-317. [Medline] [CrossRef]
- Stoupa A., Kariyawasam D., Muzza M., de Filippis T., Fugazzola L., Polak M. et al. New genetics in congenital hypothyroidism. Endocrine. 2021. 71. 696-705. [Medline] [CrossRef]
- Eugster E.A., LeMay D., Zerin J.M., Pescovitz O.H. Definitive diagnosis in children with congenital hypothyroidism. J. Pediatr. 2004. 144. 643-7. [Medline] [CrossRef]
- Unuvar T., Demir K., Abaci A., Buyukgebiz A., Bober E. The role of initial clinical and laboratory findings in infants with hyperthyrotropinemia to predict transient or permanent hypothyroidism. J. Clin. Res. Pediatr. Endocrinol. 2013. 5. 170-3. [Medline] [CrossRef]
- Rabbiosi S., Vigone M.C., Cortinovis F., Zamproni I., Fugazzola L., Persani L. et al. Congenital hypothyroidism with eutopic thyroid gland: analysis of clinical and biochemical features at diagnosis and after re-evaluation. J. Clin. Endocrinol. Metab. 2013. 98. 1395-402. [Medline] [CrossRef]
- Cho M.S., Cho G.S., Park S.H., Jung M.H., Suh B.K., Koh D.G. Earlier re-evaluation may be possible in pediatric patients with eutopic congenital hypothyroidism requiring lower L-thyroxine doses. Ann. Pediatr. Endocrinol. Metab. 2014. 19. 141-5. [Medline] [CrossRef]
- Messina M.F., Aversa T., Salzano G., Zirilli G., Sferlazzas C., De Luca F. et al. Early discrimination between transient and permanent congenital hypothyroidism in children with Eutopic gland. Horm. Res. Paediatr. 2015. 84. 159-64. [Medline] [CrossRef]
- Kara C., Gunindi F., Can Yilmaz G., Aydin M. Transient congenital hypothyroidism in Turkey: an analysis on frequency and natural course. J. Clin. Res. Pediatr. Endocrinol. 2016. 8. 170-9. [Medline] [CrossRef]
- Fu C., Luo S., Li Y., Li Q., Hu X., Li M. et al. The incidence of congenital hypothyroidism (CH) in Guangxi, China and the predictors of permanent and transient CH. Endocr. Connect. 2017. 6. 926-34. [Medline] [CrossRef]
- Park I.S., Yoon J.S., So C.H., Lee H.S., Hwang J.S. Predictors of transient congenital hypothyroidism in children with eutopic thyroid gland. Ann. Pediatr. Endocrinol. Metab. 2017. 22. 115-8. [Medline] [CrossRef]
- Zdraveska N., Zdravkovska M., Anastasovska V., Sukarova-–Angelovska E., Kocova M. Diagnostic re-evaluation of congenital hypothyroidism in Macedonia: predictors for transient or permanent hypothyroidism. Endocr. Connect. 2018. 7. 278-85. [Medline] [CrossRef]
- Saba C., Guilmin-Crepon S., Zenaty D., Martinerie L., Paul–sen A., Simon D. et al. Early determinants of thyroid function outcomes in children with congenital hypothyroidism and a normally located thyroid gland: a regional cohort study. Thyroid. 2018. 28. 959-67. [Medline] [CrossRef]
- Oron T., Lazar L., Ben-Yishai S., Tenenbaum A., Yackobovitch-Gavan M., Meyerovitch J. et al. Permanent vs transient congenital hypothyroidism: assessment of predictive variables. J. Clin. Endocrinol. Metab. 2018. 103. 4428-36. [Medline] [CrossRef]
- Higuchi S., Hasegawa Y. Levothyroxine dosages less than 2.4 pg/kg/day at 1 year and 1.3 pg/kg/day at 3 years of age may predict transient congenital hypothyroidism. Clin. Pediatr. Endocrinol. 2019. 28. 127-33. [Medline] [CrossRef]
- Itonaga T., Higuchi S., Shimura K., Nagasaki K., Satoh M., Takubo N. et al. Levothyroxine dosage as predictor of permanent and transient congenital hypothyroidism: a multicenter retrospective study in Japan. Horm. Res. Paediatr. 2019. 92. 45-51. [Medline] [CrossRef]
- Park E.S., Yoon J.Y. Factors associated with permanent hypothyroidism in infants with congenital hypothyroidism. BMC Pediatr. 2019. 19. 453. [Medline] [CrossRef]
- Asena M., Demiral M., Unal E., Ocal M., Demirbilek H., Ozbek M.N. Validity of six month L-thyroxine dose for differentiation of transient or permanent congenital hypothyroidism. J. Clin. Res. Pediatr. Endocrinol. 2020. 12. 275-80. [Medline] [CrossRef]
- Matejek N., Tittel S.R., Haberland H., Rohrer T., Busemann E.M., Jorch N. et al. Predictors of transient congenital primary hypothyroidism: data from the German registry for congenital hypothyroidism (AQUAPE “HypoDok”). Eur. J. Pediatr. 2021. 180. 2401-8. [Medline] [CrossRef]
- Yamamura H., Kokumai T., Furuya A., Suzuki S., Tanaha–shi Y., Azuma H. Increase in doses of levothyroxine at the age of 3 years and above is useful for distinguishing transient and permanent congenital hypothyroidism. Clin. Pediatr. Endocrinol. 2020. 29. 143-9. [Medline] [CrossRef]
- Mehran L., Azizi F., Mousapour P., Cheraghi L., Yarahmadi S., Amirshekari G. et al. Development of a risk prediction model for early discrimination between permanent and transient congenital hypothyroidism. Endocrine. 2021. 73. 374-83. [Medline] [CrossRef]
- Cooper D.S., Axelrod L., DeGroot L.J., Vickery A.L. Jr, Maloof F. Congenital goiter and the development of metastatic follicular carcinoma with evidence for a leak of nonhormonal iodide: clinical, pathological, kinetic, and biochemical studies and a review of the literature. J. Clin. Endocrinol. Metab. 1981. 52. 294-306. [Medline] [CrossRef]
- Medeiros-Neto G., Stanbury J. Thyroid malignancy and dyshormonogenetic goiter. Inherited disorders of the thyroid system. 1st ed. Boca Raton, FL1994. Р. 207-18.
- Drut R., Moreno A. Papillary carcinoma of the thyroid deve–loped in congenital dyshormonogenetic hypothyroidism without goiter: Diagnosis by FNAB. Diagn. Cytopathol. 2009. 37. 707-9. [Medline] [CrossRef]
- Şıklar Z., Berberoğlu M., Yağmurlu A., Hacıhamdioğlu B., Savaş Erdeve S., Fitöz S. et al. Synchronous occurrence of papillary carcinoma in the thyroid gland and thyroglossal duct in an adolescent with congenital hypothyroidism. J. Clin. Res. Pediatr. Endocrinol. 2012. 4. 30-3. [Medline] [CrossRef]
- Eremija J., Milenkovic T., Mitrovic K., Todorovic S., Vuko–vic R., Plavsic L. The first case of papillary thyroid carcinoma in an adolescent with congenital dyshormonogenetic hypothyroidism in Serbia. Vojnosanit Pregl. 2014. 71. 1078-80. [Medline] [CrossRef]
- Hishinuma A., Fukata S., Kakudo K., Murata Y., Ieiri T. High incidence of thyroid cancer in long-standing goiters with thyroglobulin mutations. Thyroid. 2005. 15. 1079-84. [Medline] [CrossRef]
- Alzahrani A.S., Baitei E.Y., Zou M., Shi Y. Clinical case semi–nar: metastatic follicular thyroid carcinoma arising from congenital goiter as a result of a novel splice donor site mutation in the thyroglobulin gene. J. Clin. Endocrinol. Metab. 2006. 91. 740-6. [Medline] [CrossRef]
- Raef H., Al-Rijjal R., Al-Shehri S., Zou M., Al-Mana H., Baitei E.Y. et al. Biallelic p.R2223H mutation in the thyroglobulin gene causes thyroglobulin retention and severe hypothyroidism with subsequent development of thyroid carcinoma. J. Clin. Endocrinol. Metab. 2010. 95. 1000-6. [Medline] [CrossRef]
- Fukata S. A case of thyroglobulin mutations (In Japanese). Journal of the Japan Thyroid Association. 2010. 1. 53-5.
- Yoon J.H., Hong A.R., Kim H.K., Kang H.C. Anaplastic thyroid cancer arising from dyshormonogenetic goiter: c.3070T>C and novel c.7070T>C mutation in the thyroglobulin gene. Thyroid. 2020. 30. 1676-80. [Medline] [CrossRef]
- Medeiros-Neto G., Gil-Da-Costa M.J., Santos C.L., Medina A.M., Silva J.C., Tsou R.M. et al. Metastatic thyroid carcinoma arising from congenital goiter due to mutation in the thyroperoxidase gene. J. Clin. Endocrinol. Metab. 1998. 83. 4162-6. [Medline]
- Chertok Shacham E., Ishay A., Irit E., Pohlenz J., Tenenbaum-Rakover Y. Minimally invasive follicular thyroid carcinoma developed in dyshormonogenetic multinodular goiter due to thyroid peroxidase gene mutation. Thyroid. 2012. 22. 542-6. [Medline] [CrossRef]
- Agretti P., Bagattini B., De Marco G., Di Cosmo C., Dionigi G., Vitti P. et al. Papillary thyroid cancer in a patient with congenital goitrous hypothyroidism due to a novel deletion in NIS gene. Endocrine. 2016. 54. 256-8. [Medline] [CrossRef]
- Camargo R., Limbert E., Gillam M., Henriques M.M., Fernandes C., Catarino A.L. et al. Aggressive metastatic follicular thyroid carcinoma with anaplastic transformation arising from a long-standing goiter in a patient with Pendred’s syndrome. Thyroid. 2001. 11. 981-8. [Medline] [CrossRef]
- Sakurai K., Hata M., Hishinuma A., Ushijima R., Okada A., Taeda Y. et al. Papillary thyroid carcinoma in one of identical twin patients with Pendred syndrome. Endocr. J. 2013. 60. 805-11. [Medline] [CrossRef]
- Nikiforov Y.E., Nikiforova M.N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011. 7. 569-80. [Medline] [CrossRef]
- McLeod D.S., Watters K.F., Carpenter A.D., Ladenson P.W., Cooper D.S., Ding E.L. Thyrotropin and thyroid cancer diagnosis: a systematic review and dose-response meta-analysis. J. Clin. Endocrinol. Metab. 2012. 97. 2682-92. [Medline] [CrossRef]
- Francis G.L., Waguespack S.G., Bauer A.J., Angelos P., Benvenga S., Cerutti J.M. et al. American Thyroid Association Guidelines Task Force. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015. 25. 716-59. [Medline] [CrossRef]
- Zheng J., Li C., Lu W., Wang C., Ai Z. Quantitative assessment of preoperative serum thyrotropin level and thyroid cancer. Oncotarget. 2016. 7. 34918-29. [Medline] [CrossRef]
- Hishinuma A. Thyroglobulin gene abnormalities. Rinsho Byori. 2005. 53. 935-41 (in Japanese). [Medline]
- Hishinuma A., Ieiri T., Fukata S., Miyauchi A. Thyroglo–bulin gene abnormalities. Nihon Rinsho. 2005. 63 (Suppl. 10). 31-5 (in Japanese). [Medline]
- De Jaco A., Dubi N., Camp S., Taylor P. Congenital hypothyroidism mutations affect common folding and trafficking in the o/p-hydrolase fold proteins. FEBS J. 2012. 279. 4293-305. [Medline] [CrossRef]
- Kryston T.B., Georgiev A.B., Pissis P., Georgakilas A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. 2011. 711. 193-201. [Medline] [CrossRef]
- Ameziane El Hassani R., Buffet C., Leboulleux S., Dupuy C. Oxidative stress in thyroid carcinomas: biological and clinical significance. Endocr. Relat. Cancer. 2019. 26. R131-43. [Medline] [CrossRef]
- Hamburger J.I., Hamburger S.W. Thyroidal hemiagenesis. Report of a case and comments on clinical ramifications. Arch. Surg. 1970. 100. 319-20. [Medline] [CrossRef]
- Greening W.P., Sarker S.K., Osborne M.P. Hemiagenesis ofthe thyroid gland. Br. J. Surg. 1980. 67. 446-8. [Medline] [CrossRef]
- Massine R.E., Durning S.J., Koroscil T.M. Lingual thyroid carcinoma: a case report and review of the literature. Thyroid. 2001. 11. 1191-6. [Medline] [CrossRef]
- Sturniolo G., Violi M.A., Galletti B., Baldari S., Campenni A., Vermiglio F. et al. Differentiated thyroid carcinoma in lingual thyroid. Endocrine. 2016. 51. 189-98. [Medline] [CrossRef]

/76.jpg)
/81.jpg)
/82.jpg)
/84.jpg)